Introduction
PCM (Phase Change Materials) are materials which are used as latent-heat storage systems. The solid-to-liquid Phase TransitionsThe term phase transition (or phase change) is most commonly used to describe transitions between the solid, liquid and gaseous states.phase transition enthalpy is thereby used for heat storage. The application field for latent-heat storage systems spans from pocket heaters to functional textiles to wall and ceiling elements in building construction. The thermophysical properties of a PCM sample of plant extracts were investigated with the help of the LFA 467 HyperFlash® and DSC 204 F1 Phoenix®.
Test Conditions
LFA:

- 30°C to 150°C solid sample in the standard sample holder (heating)
- 220°C to 30°C liquid sample in the PEEK sample holder (cooling), see figure 1
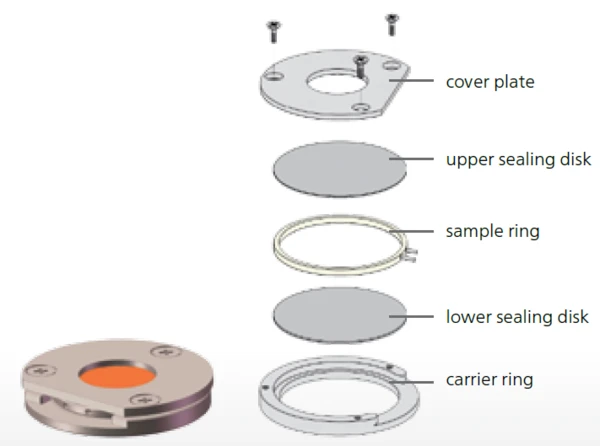
DSC:

- -10°C to 225°C heating and cooling
Measurement Results
Figure 2 shows the heating and cooling of the PCM sample by means of DSC. Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).Melting of the sample starts at approx. 165°C (onset), crystallization during cooling, however, only starts again at approx. 123°C. This effect can also be seen for the LFA measurements. The red squares in figure 3 represent the Diffusività TermicaThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity of the PCM sample during cooling (from liquid to solid). The step in the the thermal diffusivity can be related to the Phase TransitionsThe term phase transition (or phase change) is most commonly used to describe transitions between the solid, liquid and gaseous states.phase transition. Since the measuring points were recorded during cooling, the phase transiton appears between 120°C and 150°C. The red triangles in figure 3 represent the thermal diffusivity during heating of the PCM sample. Both measurements are in good agreement with one another. Only at 150°C can a significant difference be seen, which is attributable to the different states of the samples (liqud and solid) resulting from the differing melting and crystallization temperatures.
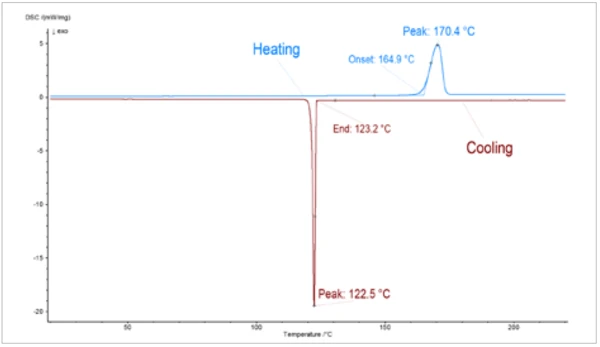
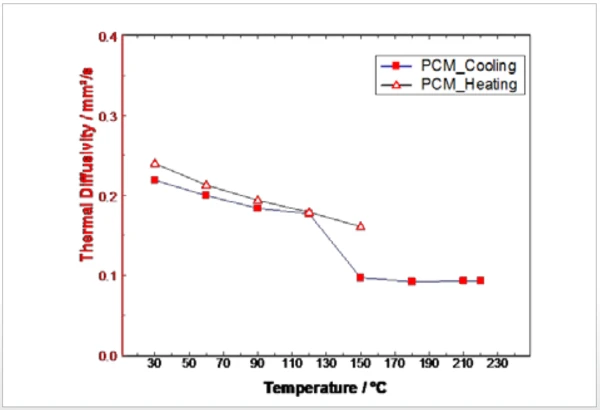
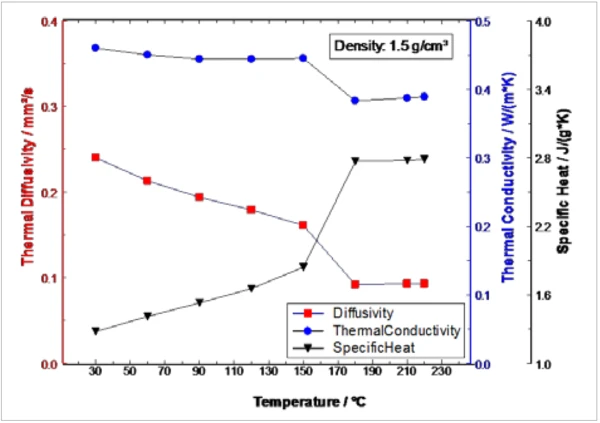
Figure 4 shows the thermophysical properties duirng heating of the PCM sample between 30°C and 220°C as a combination of the two measurements. The solid-to-liquid Phase TransitionsThe term phase transition (or phase change) is most commonly used to describe transitions between the solid, liquid and gaseous states.phase transition can be clearly identified in the thermal diffusivity as well as in the Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity and the Conduttività TermicaThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity by means of a step between 150°C and 180°C.
Summary
The special sample holder for liquids and pastes (PEEK sample holder) allows investigation of the thermal diffusivity of PCM samples even into the melt, by means of LFA. Comparative measurements with and without the liquid sample holder in the solid range are in good agreement as long as there is good contact between the sample and sample holder (3-layer analysis). DSC measurements allow conclusions to be drawn on the melting and crystallization behavior of the samples and yield data on the Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity. From the measurements of both methods, reliable statements about the thermal conductivity of the PCM sample in the solid and liquid range can subsequently be made.