Determination of the Battery Condition
When it comes to using an energy storage unit, its current “fill level” is always of interest – be it for evaluating the remaining run time of a mobile phone or a laptop, or with regard to the range of an electric vehicle. Though the charging time may play a rather minor role for a cellphone or laptop, it can be of particular importance in the context of electromobility.
Describing the current state of an energy storage unit well can be more difficult than it first appears. A good illustration of the current state of an accumulator is the barrel model [1]. This model has already been described in detail in connection with the cycling of coin cells [2]. In the following, the heat development during charging and discharging of 18650 cells, i.e., significantly larger batteries than coin cells, will be investigated.
The NETZSCH ARC® 254
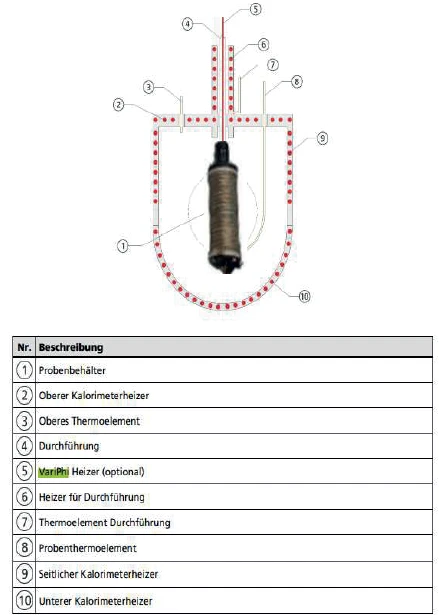
The NETZSCH ARC® 254 (figure 1) is an Accelerating Rate Calorimeter, an instrument which is usually used to investigate the so-called Thermal runawayA thermal runaway is the situation where a chemical reactor is out of control with respect to temperature and/or pressure production caused by the chemical reaction itself. Simulation of a thermal runaway is usually carried out using a calorimeter device according to accelerated rate calorimetry (ARC).thermal runaway of individual substances or reaction mixtures [3]. With regard to the cycling of batteries, however, the ARC® 254 is to be used as an isothermal calorimeter. To this end, the setup of the ARC® 254 can be used in a special way. For the abovementioned safety investigations, the actual calorimeter chamber in the ARC® 254 is surrounded by various independent heaters. For the isothermal examination of accumulators, these are enclosed by another heater in the calorimeter, so that the temperature of the battery can be controlled independently of the calorimeter.
18650 Cells
So-called 18650 cells are standard industry cells in a cylindrical metal housing with a diameter of 18 mm and a height of 65.0 mm (figure 2).
The battery is placed into a heater surrounding the cylindrical cell (figure 3) and installed in the measuring chamber of the calorimeter.
The battery is connected with the external cycling unit (figure 4) via a simple connector plug in order to apply current and voltage for charging and discharging.
The interest in determining the thermal balances of batteries during charging and discharging, while a top current issue, is not entirely new. Although the setup in the NETZSCH ARC® 254 described below differs from the templates in literature, the basic approach is identical to the one described by Hansen et al. in 1982 [4].


H2Secure
heater
The 3D-H2Secure
Heater
As already indicated, the cylindrical battery is directly surrounded by the 3D- H2Secure
To create a sufficiently long control system, the other heaters of the calorimeter (2 , 6 , 9 and 10 in figure 5) are set to a constant lower temperature. If the energetic processes during charging and discharging in the battery were to change the temperature of the cell, the power supply of the 3D- H2Secure H2Secure
Since the power required by the 3D- H2Secure
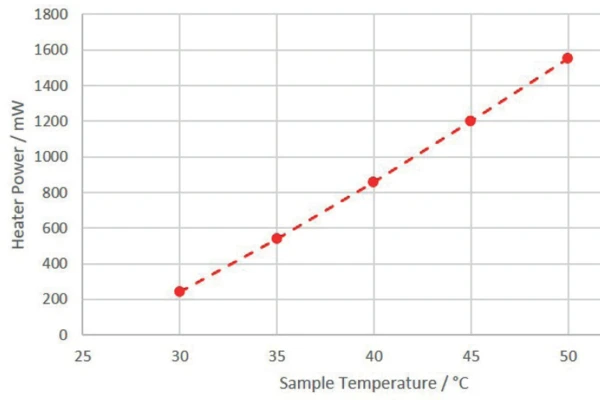
H2Secure
heater to realize the correspoding sample temperature versus 25°C calorimeter temperatureCycling of a 18650 Cell
The 18650 cell to be investigated was kept at a constant temperature of 35°C by the 3D- H2Secure
Table 1: Charging and discharging currents
| Charging | Discharging | |
| 1C | 1500 mA | 1500 mA |
| C/2 | 750 mA | 750 mA |
| C/4 | 375 mA | 375 mA |
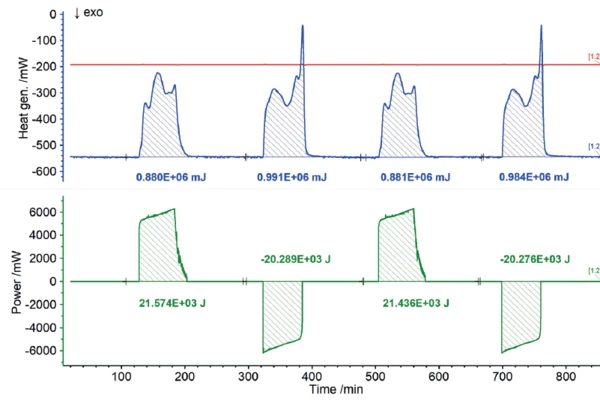
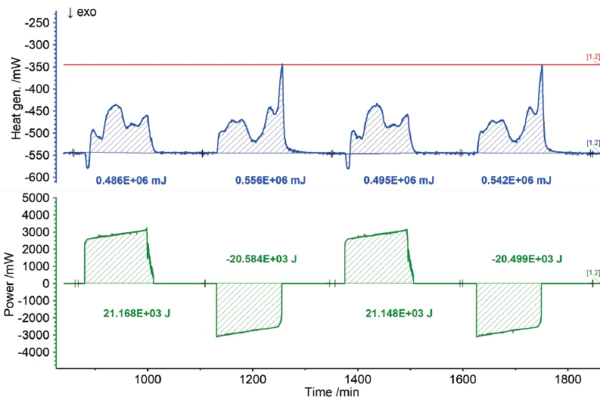
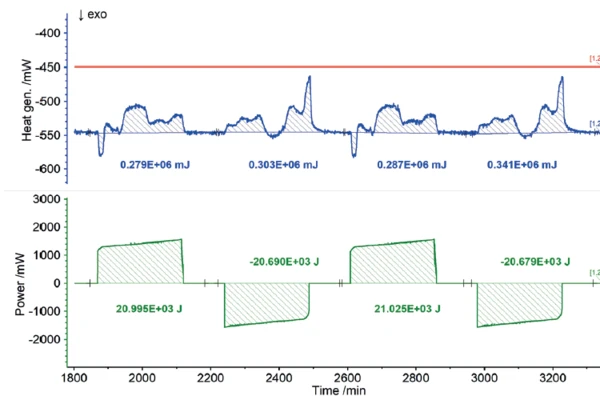
Users all know from their own experience that mobile phones or laptops heat up during intensive operation and likewise during charging. In terms of the charging cycle, these heat developments represent energy losses, because the portion of heat released this way is not available for actual use by the energy storage unit. Consequently, the heat quantities detected by the ARC® 254 during charging and discharging can be recorded as losses in terms of charging efficiency. The results for the heat of reaction of the 18650 cell as a function of different charging rates are shown in figures 7 to 9. If the charging or discharging power invested is compared with the measured heats of reaction, i.e., the losses, the efficiency of the partial cycles can be determined independently.
Summary
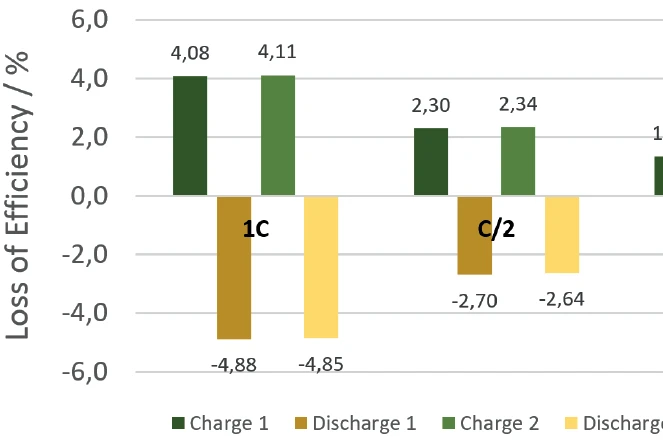
The NETZSCH ARC® 254 was used to cycle a cylindrical battery (18650) at 35°C at different charging rates (1C, C/2, C/4). The detected heats of reaction correspond to the thermal losses, which allow the efficiency of the cycles for charging and discharging to be determined independently of each other. If there were no losses, the efficiency would be 100%. The losses determined from the heats of reaction are summarized for the charging and discharging cycles, but also for the different charge rates, in figure 10. It is clear that for low charge rates (C/4), the losses are lower and thus the efficiency is higher than for higher charge rates (1C).
