Inherently Safe Process Control
Reactions must remain controllable at all times, not only on a laboratory scale of usually less than one liter, but also – and especially – in large reactors that produce on a ton scale. Even if unplanned events like the failure of a pump in the cooling cycle of a reactor occur, precautions need to already have been taken at the planning stage of the production plant to prevent reactors from getting out of control. This forward-looking planning, which also takes unforeseeable events into account, enables inherently safe operation of production plants at all times [1].
Worst-Case-Scenario
Already before planning production plants, it is essential to assess the chemicals used and the planned reactions in terms of their hazard potential. In order to avoid unpleasant surprises in the size and capacity of the plants, in upscaling, or even in the order in which the reactants are added, studies are often carried out to this end describing the Worst-Case ScenarioRelated to a chemical reactor, a worst-case scenario is the situation where temperature and/or pressure production caused by the reaction runs out of control.worst-case scenario. Knowledge of the worst case makes it easier to control all real production conditions. The worst case with regard to the temperature control of a reactor is exceeding the planned process temperature due to, for example, the failure of a pump in the cooling cycle. If the cooling system fails and the heat of reaction can no longer be balanced, the temperature in the reactor rises above the planned reaction temperature. This can lead to undesirable side reactions or secondary reactions. In the worst case, temperature and/or pressure increases can cause the reactor to burst. In order to investigate what happens when the temperature in the reactor rises uncontrollably, how fast the temperature rises and how much pressure builds up in the reactor, such reactions are simulated on a small scale in the laboratory. An instrument designed to investigate this worst case is the NETZSCH Calorimetria por taxa de Aceleração (ARC)The method describing isothermal and adiabatic test procedures used to detect thermally exothermic decomposition reactions.ARC® 254.
The NETZSCH ARC® 254
The NETZSCH ARC® 254 (figure 1) is an accelerating rate calorimeter capable of carrying out so-called Thermal runawayA thermal runaway is the situation where a chemical reactor is out of control with respect to temperature and/or pressure production caused by the chemical reaction itself. Simulation of a thermal runaway is usually carried out using a calorimeter device according to accelerated rate calorimetry (ARC).thermal runaway tests. The objective of this measurement technology is to find the hazardous potential with respect to the temperature of a sample or a reaction mixture under AdiabáticoAdiabatic describes a system or measurement mode without any heat exchange with the surroundings. This mode can be realized using a calorimeter device according to the method of accelerating rate calorimetry (ARC). The main purpose of such a device is to study scenarios and thermal runaway reactions. A short description of the adiabatic mode is “no heat in – no heat out”.adiabatic conditions. Adiabaticity in particular means no heat exchange occurs. If all the heat of reaction remains inside a reaction vessel and is not able to dissipate to the environment, the temperature will rise and thus cause the speed of reaction to increase. This will result in a self-accelerating reaction mechanism. By studying such scenarios, any real-world conditions – which as a rule are not fully adiabatic, since some heat is always lost to the surroundings – can be calculated and classified.

How is the Detection of an Exothermal Self-DecompositionReaction Detected?
To detect Thermal runawayA thermal runaway is the situation where a chemical reactor is out of control with respect to temperature and/or pressure production caused by the chemical reaction itself. Simulation of a thermal runaway is usually carried out using a calorimeter device according to accelerated rate calorimetry (ARC).thermal runaway, the temperature of the substance or reaction mixture to be investigated is increased stepwise. At each temperature step, a sufficient amount of time is waited in order to temper the sample to that temperature. Then, a detection is carried out as to whether the sample temperature remains constant at this temperature or whether it rises slowly, i.e., whether self-heating of the sample occurs or not. If no self-heating is detected, this sequence of stepwise temperature increase (Calor – Esperar – PesquisaHeat-Wait-Search is a measurement mode used in calorimeter devices according to accelerating rate calorimetry (ARC).Heat-Wait-Search) will be continued. When exceeding the Self-heating rateA special kind of calorimeter device is used in order to detect the self-heating rate of a substance. The related method is called accelerating rate calorimetry (ARC). self-heating rate of 0.02 K/min, the instrument changes into the so-called adiabatic mode. This measurement mode prevents heat loss to the sample environment, since all heaters surrounding the sample chamber follow the sample temperature now. If all heaters have the same temperature as the sample, i.e., there is no temperature gradient, no heat can be lost to the environment. This way, the ARC® ensures an adiabatic sample environment to the greatest extent possible. This, in turn, is an important prerequisite for investigating a Worst-Case ScenarioRelated to a chemical reactor, a worst-case scenario is the situation where temperature and/or pressure production caused by the reaction runs out of control.worst-case scenario such as Thermal runawayA thermal runaway is the situation where a chemical reactor is out of control with respect to temperature and/or pressure production caused by the chemical reaction itself. Simulation of a thermal runaway is usually carried out using a calorimeter device according to accelerated rate calorimetry (ARC).thermal runaway.
How is a Thermal Runaway Reaction Measured?
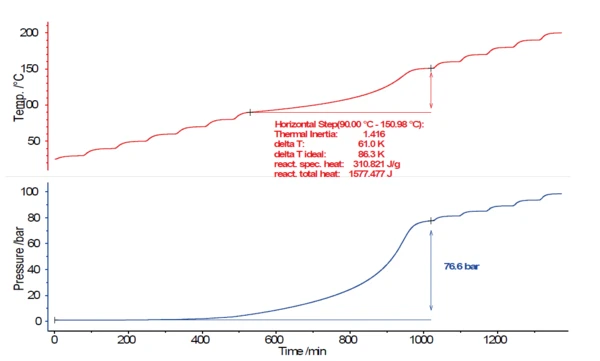
If Thermal runawayA thermal runaway is the situation where a chemical reactor is out of control with respect to temperature and/or pressure production caused by the chemical reaction itself. Simulation of a thermal runaway is usually carried out using a calorimeter device according to accelerated rate calorimetry (ARC).thermal runaway begins to occur during a reaction, it is desirable to determine this critical point in time or temperature as early as possible. Carried out sequentially, the sample temperature will initially increase only very slowly at the beginning of self-heating. 0.02 K/min is a very low Self-heating rateA special kind of calorimeter device is used in order to detect the self-heating rate of a substance. The related method is called accelerating rate calorimetry (ARC). self-heating rate, corresponding to only 1.2 K per hour. The Reação de DecomposiçãoA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition reaction starts slowly, but continuously increases in speed with rising temperature until it reaches its maximum Self-heating rateA special kind of calorimeter device is used in order to detect the self-heating rate of a substance. The related method is called accelerating rate calorimetry (ARC). self-heating rate and finally the maximum temperature. Figure 3 shows the results for temperature (red) and pressure (blue) for an Calor – Esperar – PesquisaHeat-Wait-Search is a measurement mode used in calorimeter devices according to accelerating rate calorimetry (ARC).HWS test on a 17.5% hydrogen peroxide solution (H2O2). To this end, a volume of 5.0757 g of the hydrogen peroxide solution was placed into a spherical titanium container (8.7 ml).
As mentioned earlier, the criterion for recognizing an ExotérmicoA sample transition or a reaction is exothermic if heat is generated.exothermal decomposition reaction is a Self-heating rateA special kind of calorimeter device is used in order to detect the self-heating rate of a substance. The related method is called accelerating rate calorimetry (ARC). self-heating rate of > 0.02 K/min. This threshold value was exceeded at 90°C (onset), and then the sample temperature rose to 151°C under adiabatic conditions. During the decomposition reaction, the pressure inside the sample vessel increased to 76.6 bar.
Is There a Way to Stop Thermal Runaway?
The question as to whether Thermal runawayA thermal runaway is the situation where a chemical reactor is out of control with respect to temperature and/or pressure production caused by the chemical reaction itself. Simulation of a thermal runaway is usually carried out using a calorimeter device according to accelerated rate calorimetry (ARC).thermal runaway can be stopped or not is, of course, strongly related to the selfheating rate. It is necessary to detect the critical temperature or the start of Thermal runawayA thermal runaway is the situation where a chemical reactor is out of control with respect to temperature and/or pressure production caused by the chemical reaction itself. Simulation of a thermal runaway is usually carried out using a calorimeter device according to accelerated rate calorimetry (ARC).thermal runaway, but maybe it is not always desirable to allow the decomposition reaction to fully run its course. It would be much more important to know the temperature or pressure up to which a reaction that has already begun to undergo runaway can be stopped again and brought under control. The possibility of detecting the beginning of a reaction’s Thermal runawayA thermal runaway is the situation where a chemical reactor is out of control with respect to temperature and/or pressure production caused by the chemical reaction itself. Simulation of a thermal runaway is usually carried out using a calorimeter device according to accelerated rate calorimetry (ARC).thermal runaway and then preventing further self-heating by shutting off the adiabatic environment, thus avoiding the decomposition reaction, has already been reported about elsewhere [2]. Here, an attempt should be made to show another way to stop a decomposition reaction that has just started by pursuing a different strategy. The reaction vessel is connected via a pressure line and a valve to another vessel, the so-called vent vessel (figure 3). When a freely selectable sample pressure is reached, the measuring software will open the valve to the vent vessel. By venting into this vessel, the pressure in the reaction vessel should also decrease. This could be sufficient to stop self-heating and thus uncontrolled consecutive and side reactions.
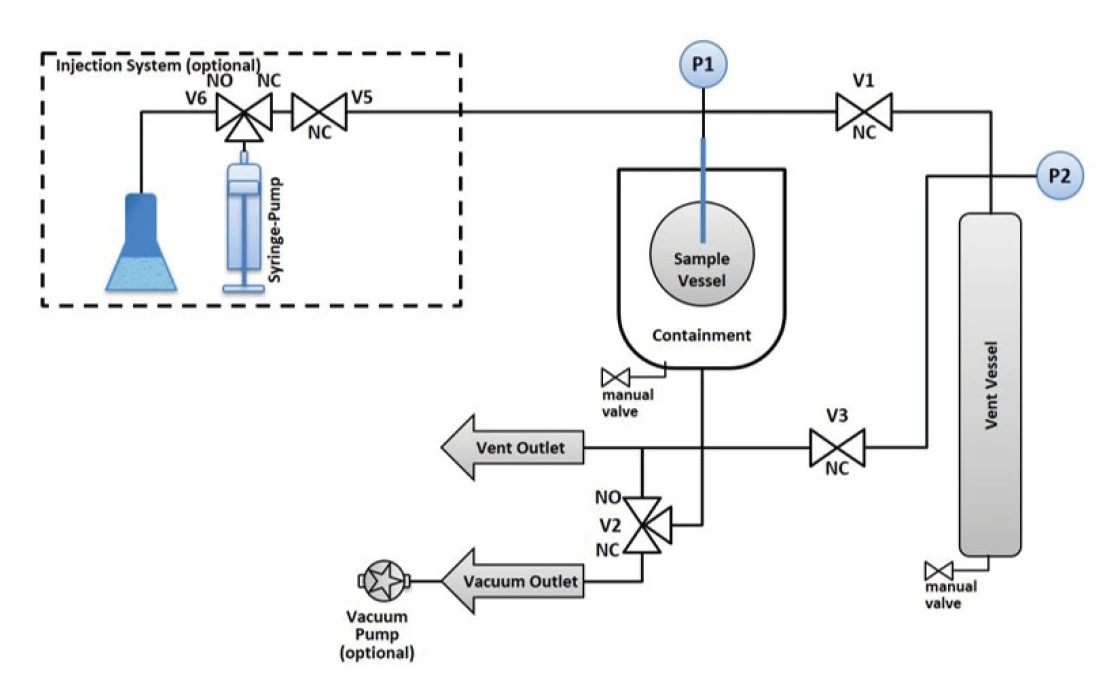
Venting
Both the reaction vessel and vent vessel are equipped with an indivdual pressure gauge. Thus, the pressure increase can be tracked after opening the valve (see V1 in figure 3). The volume of the vent vessel at 250 ml, however, is many times larger than the volume of the sample vessel, where typically approx. 5 ml of gas volume remain above the sample. For this reason, the pressure in the vent vessel increases only from 1.0 bar to 1.13 bar after opening the valve, while the pressure in the sample vessel decreases from 10.0 bar to 1.0 bar at the same time (figure 4).
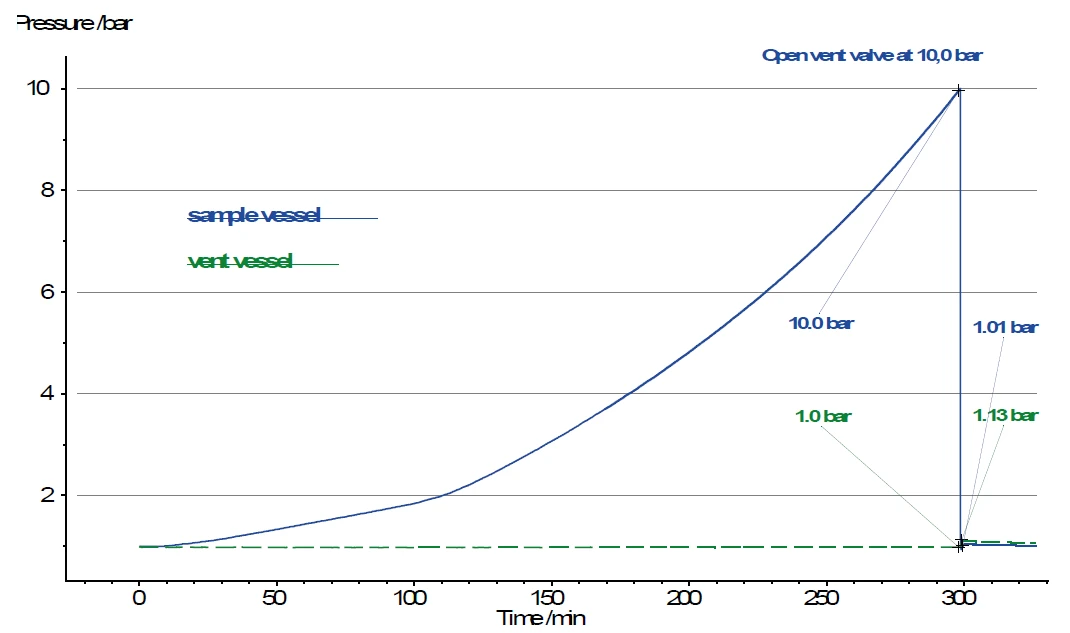
Figure 5 shows the results of an HWS measurement with water as the sample substance, in which the pressure signal increases analogously to the temperature signal and according to the temperature steps of the HWS program. In this example, opening of the vent valve was programmed via the measurement software at 2.0 bar. It can easily be seen that by opening the vent vessel, not only does the pressure in the sample vessel decrease from 2.0 bar to 1.0 bar, but the temperature in the sample vessel also strongly decreases. Over a period of 60 min in which the vent valve remains open, the heaters surrounding the calorimeter also follow the sample temperature. This decreases from 108.4°C to 96.8°C and – although the adiabatic measurement mode remains activated during this time, i.e., the surrounding heaters follow the sample temperature – no further increase in sample temperature can be determined.
Now, when investigating water as a sample substance, it can be expected that there will be no exothermal reaction. Instead, it was confirmed that when there is no exothermal reaction by the sample, the sample temperature decreases after opening the vent valve and then remains constant due to the adiabatic surrounding. This is also confirmed by the Self-heating rateA special kind of calorimeter device is used in order to detect the self-heating rate of a substance. The related method is called accelerating rate calorimetry (ARC). self-heating rate of the sample in the lower part of the figure.
The investigation of a one-percent hydrogen peroxide solution also shows no further temperature increase after opening the vent valve at a pressure of 3 bar in the sample vessel. In the case of a two-percent hydrogen peroxide solution, it can already be seen that the exothermal decomposition reaction caused by opening the vent valve and depressurizing the system to atmospheric pressure is not sufficient to entirely suppress further decomposition. This results in a Self-heating rateA special kind of calorimeter device is used in order to detect the self-heating rate of a substance. The related method is called accelerating rate calorimetry (ARC). self-heating rate of 0.02 K/min. For a four-percent hydrogen peroxide solution (figure 6), a Self-heating rateA special kind of calorimeter device is used in order to detect the self-heating rate of a substance. The related method is called accelerating rate calorimetry (ARC). self-heating rate of 0.04 K/min is still detected after opening the vent valve. The temperatures and self-heating rates for the hydrogen peroxide solutions discussed are summarized in table 1.
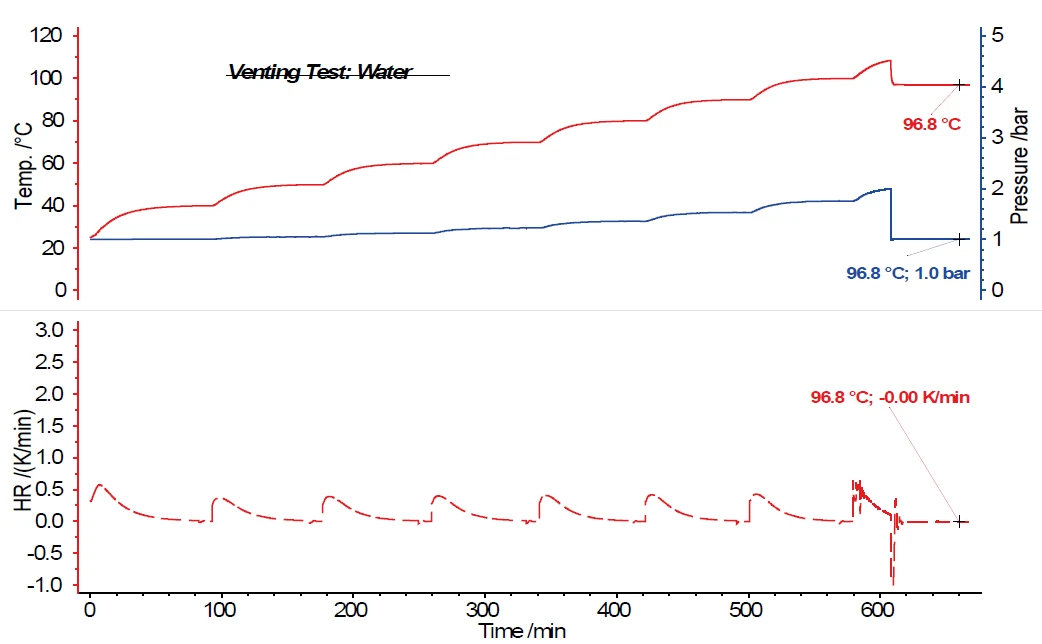
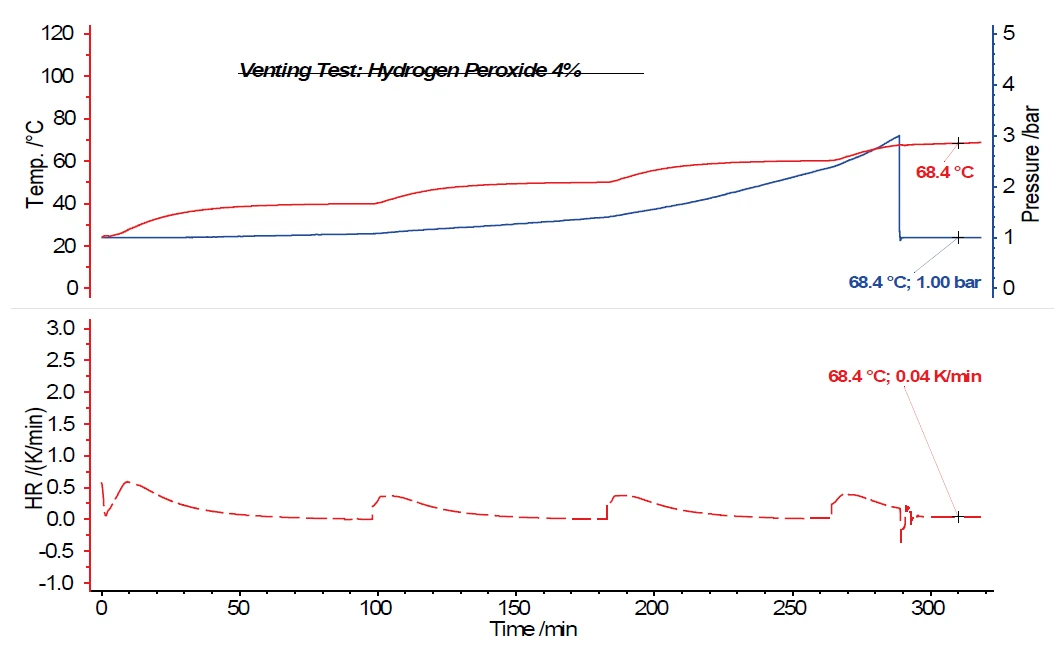
Table 1: Summary of the temperature and Self-heating rateA special kind of calorimeter device is used in order to detect the self-heating rate of a substance. The related method is called accelerating rate calorimetry (ARC). self-heating rate for various hydrogen peroxide solutions
| Sample | Temperature during venting | Self-heating rateA special kind of calorimeter device is used in order to detect the self-heating rate of a substance. The related method is called accelerating rate calorimetry (ARC). Self-heating rate after venting |
| H2O | 108.4°C (2 bar) | 0.00 K/min |
| H2O2 (1%) | 81.8°C (3 bar) | 0.00 K/min |
| H2O2 (2%) | 70.8°C (3 bar) | 0.02 K/min |
| H2O2 (4%) | 67.6°C (3 bar) | 0.04 K/min |
Summary
The NETZSCH ARC® 254 offers two possibilities for regaining control, if necessary, of reactions where Thermal runawayA thermal runaway is the situation where a chemical reactor is out of control with respect to temperature and/or pressure production caused by the chemical reaction itself. Simulation of a thermal runaway is usually carried out using a calorimeter device according to accelerated rate calorimetry (ARC).thermal runaway has already started. One possibility is that in which the surrounding heaters are switched off when the sample reaches a given Self-heating rateA special kind of calorimeter device is used in order to detect the self-heating rate of a substance. The related method is called accelerating rate calorimetry (ARC). self-heating rate, thus eliminating the adiabatic environment of the sample and making heat losses possible again; further runaway of the reaction is then staved off via these heat losses [2]. The other possibility, in which pressure can be removed from the sample vessel into another sample vessel (vent vessel) by opening a pressure relief valve (vent valve), was presented in this application note. By measuring the pressure independently, the pressure increase in the vent vessel can be monitored. It was shown that further progress in weakly exothermal Reação de DecomposiçãoA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition reactions can be stopped by this, while more strongly exothermal reactions continue to show detectable self-heating even after the pressure has been released.