Introduction
Eudragit® is the trade name of polymethacrylate-based co-polymers used to target the release of a drug in the desired parts of the gastro-intestinal tract. Eudragit® exists in several compositions that differ from each other in terms of the functional groups located on the side chains.
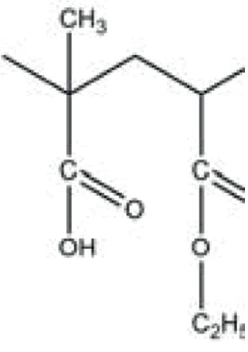
This results in differing dissolution behavior depending on the pH value of the environment. For example, Eudragit® L100-55 (figure 1) is soluble in intestinal fluids from pH 5.5 upward, but is not soluble in gastric fluids with lower pH values. Thus, it is used in combination with drugs to be released in the duodenum after passing the stomach [1, 2, 3].
Thermal analysis of Eudragit® products is crucial for different reasons: Firstly, they differ from each other in their Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition temperature (Tg). Even Eudragit® polymers with a similar chemical composition show differences in Tg, depending on the monomer ratios [1]. Thus, the determination of Tg allows for identification of the different Eudragit® polymers. Secondly, optimum process conditions, e.g., for hot melt extrusion, require knowledge of the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition temperature and Thermal StabilityA material is thermally stable if it does not decompose under the influence of temperature. One way to determine the thermal stability of a substance is to use a TGA (thermogravimetric analyzer). thermal stability of the polymer [3].
For this reason, the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition process of Eudragit® L100-55 (Evonik Industries) is investigated by means of a thermobalance (TGA) coupled to an FT-IR spectrometer.
Measurement Conditions
The TGA-FT-IR measurement was performed using a NETZSCH TG 209 F1 Libra® thermobalance. To investigate and identify the gases released during thermogravimetric analysis, they were transferred directly into the gas cell of an FT-IR system by Bruker Optics. The measurement was carried out on 7.33 mg of Eudragit® L100-55, using an open aluminum oxide crucible.
The sample was heated between room temperature and 600°C at 10 K/min in a nitrogen atmosphere (40 ml/min).
Measurement Results
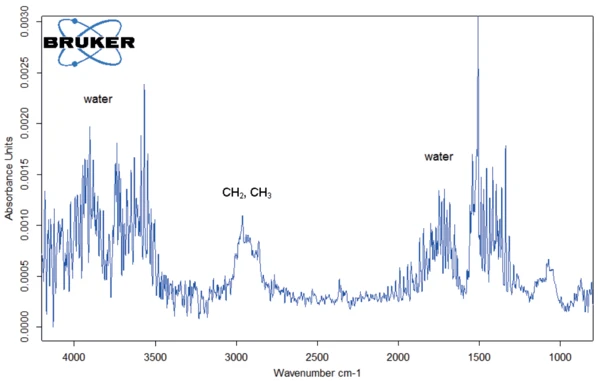
Figure 2 depicts the mass changes of Eudragit® L100-55 between 40°C and 600°C. The first mass-loss step of 0.8% indicates the release of surface water up to 100°C. The second mass loss of 5.9% at 200°C (DTG peak) is also associated with the release of water confirmed by the FT-IR spectrum (figure 3). The temperature of the process indicates the release of crystal water. In addition, bands occur in the wavelength range 3000 - 2800 cm-1 and above 1000 cm-1. These bands represent CH2 and CH3 molecules that indicate the start of Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of the Eudragit® sample.
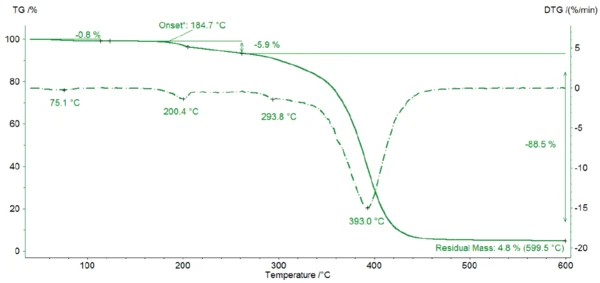
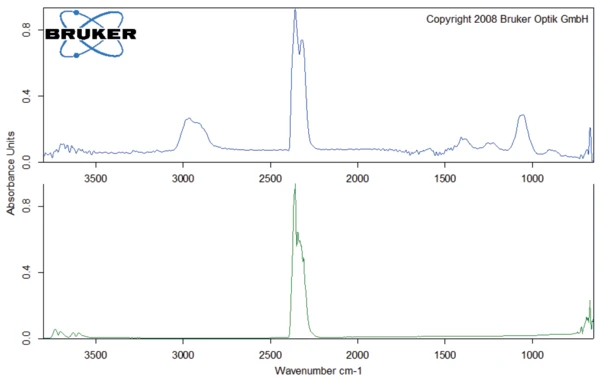
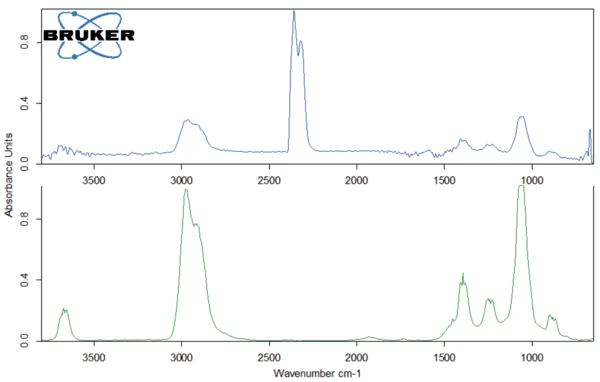
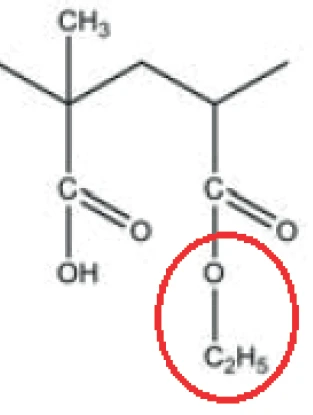
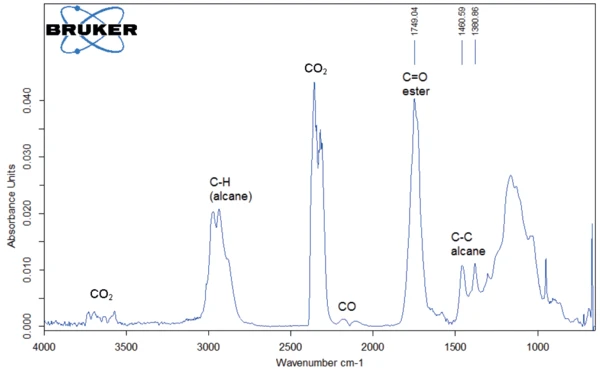
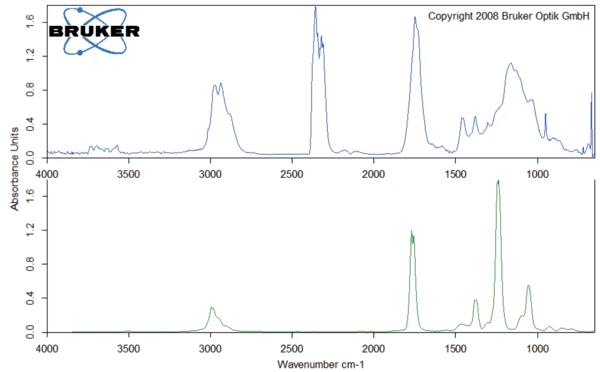
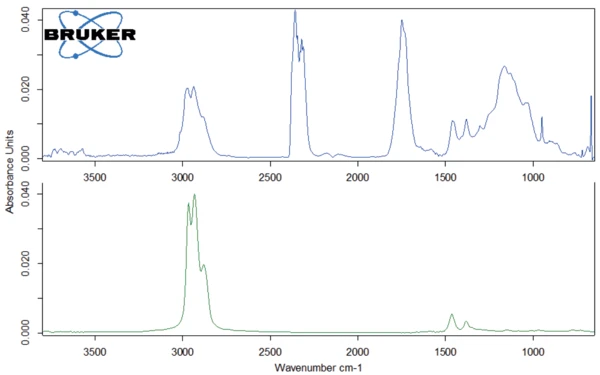
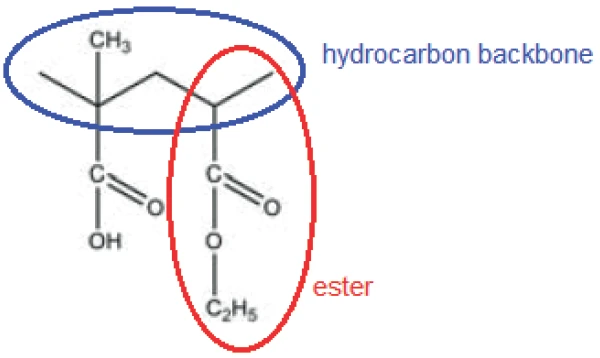
The peak at 294°C in the DTG curve is associated with yet another step in the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition process: the release of carbon dioxide and probably ethanol (figures 4 and 5). This can be explained by the splitting of an ester group off the Eudragit® molecule (figure 6). The last and main Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition step, with a mass loss of 88.5%, occurs at 393°C (DTG peak temperature). The characteristic bands of ethanol and of carbon dioxide can still be detected in the FT-IR-spectrum of the gases released at 393°C (figure 7). In addition, carbon monoxide (2300 cm-1 to 2100 cm-1) and an ester substance are present, which can be seen in the carbonyl band at 1749 cm-1. It suggests that the ester part C2H5-O-CO-CxHy breaks off from the molecule (see red indication in figure 10). The two VibrationA mechanic process of oscillation is called vibration. Vibration is a mechanical phenomenon whereby oscillations occur about an equilibrium point. In many cases, vibration is undesirable, wasting energy and creating unwanted sound. For example, the vibrational motions of engines, electric motors, or any mechanical device in operation are typically unwanted. Such vibrations could be caused by imbalances in the rotating parts, uneven friction, or the meshing of gear teeth. Careful designs usually minimize unwanted vibrations.vibration bands at 1460 cm-1 and 1380 cm-1 are probably due to parts of the carbon backbone. As an example, a comparison spectrum of ethyl acetate and 3-methyloctane is illustrated in figures 8 and 9.
Conclusion
The start of Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of Eudragit® is closely related to the Thermal StabilityA material is thermally stable if it does not decompose under the influence of temperature. One way to determine the thermal stability of a substance is to use a TGA (thermogravimetric analyzer). thermal stability. It results in changes in sample mass during storage or thermal treatment. The mass changes can be identified by means of thermogravimetry. However, clear identification of the released gases – and thus a reliable interpretation of the mass losses – is only possible when the thermobalance is coupled to an FT-IR device. This allows for reliable conclusions to be drawn as to whether a given mass loss can be attributed to decomposition or merely to the release of water.
Under the selected conditions (inert atmosphere, heating rate of 10 K/min), the investigated Eudragit® sample starts to decompose at 185°C (onset temperature of the TGA curve). The fact that this is really the start of decomposition is revealed by the occurrence of C-H bonds in addition to crystal water.