Introduction
Eudragit® is the trade name of polymethacrylate-based co-polymers used to target the release of a drug in the desired parts of the gastro-intestinal tract. Eudragit® exists in several compositions that differ from each other in the functional groups located on the side chains. The chemical composition of the monomers and the molecular weight or the chain length of the entire polymer have a major influence on its Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition temperature [4].
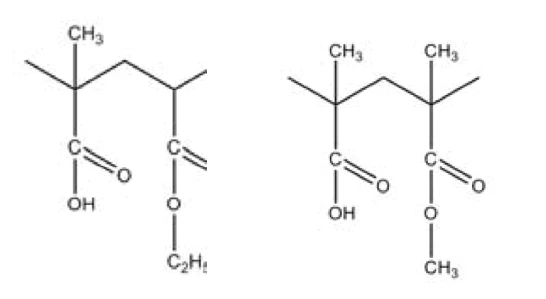
As an example, figure 1 depicts two repeating units of Eudragit® polymers that are very similar in chemical composition. However, the polymers differ greatly in their Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition temperature: It is 111°C for Eudragit® L100-55 and 195°C for Eudragit® L100 (onset temperatures of the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition, see [1]). This explains the fact that the primary objective in the quality control of Eudragit® polymers is the determination of the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition temperature for reliable identification of the relevant product.
Secondly, knowledge of the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition temperature of the polymer is needed for selection of the optimum process conditions for certain processes, e.g., hot-melt extrusion [3].
In the following, an unidentified Eudragit® polymer is identified with differential scanning calorimetry (DSC).
Limits of Standard DSC Measurements
The measurement was carried out on 3.38 mg of Eudragit® L100-55, using a closed aluminum crucible with pierced lid. The sample was heated from -50°C to 250°C at 10 K/min in a nitrogen atmosphere (40 ml/min) with the DSC 204 F1 Nevio.
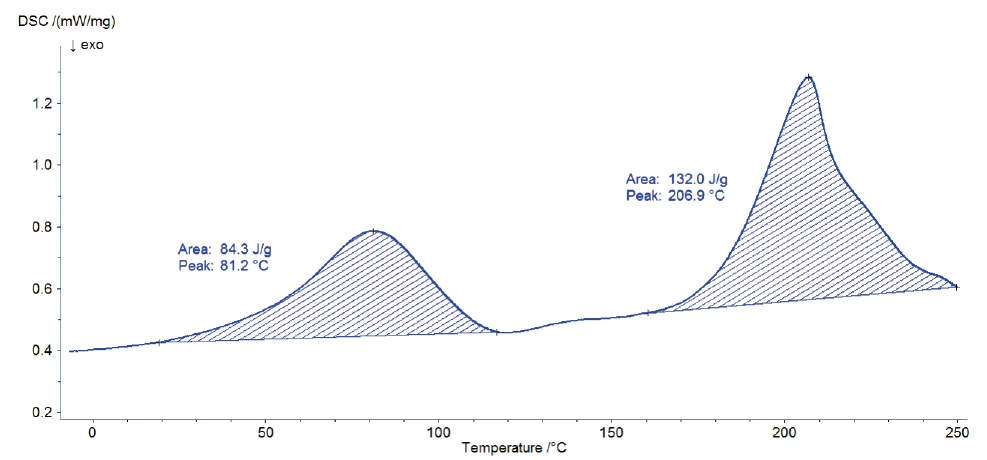
Figure 2 depicts the DSC curve during heating. Two endothermal peaks with peak temperatures at 81°C and 207°C were detected. The position and the shape of the first peak indicate evaporation of water. This result was confirmed by means of TG-FT-IR measurements on the sample [5]. The second peak is probably due to the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of the sample [1]. An endothermal step is located between the two peaks, suggesting the glass transition of the substance. However, accurate evaluation of it requires separation of this effect from the evaporation peak. For that, a modulated DSC measurement is carried out.
For an Accurate Evaluation of Tg: Modulated DSC
A mass of 4.80 mg of the unidentified Eudragit® was weighed and measured with the DSC 204 F1 Nevio. The sample was heated from -40°C to 180°C. The temperature increase was not linear, but oscillated with an amplitude of 0.5 K and a period of 60 s. The oscillations were superimposing an underlying heating rate of 3 K/min.
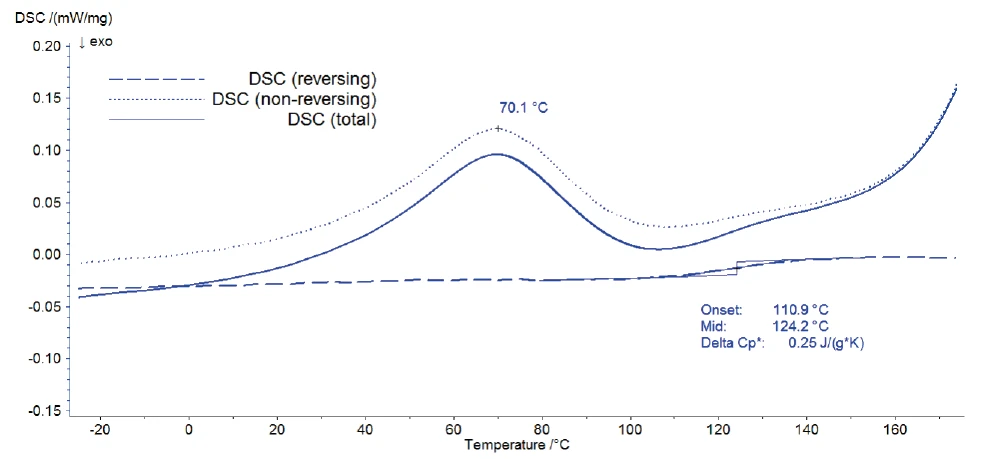
Figure 3 shows the result of the modulation: The total DSC signal provides information about all the processes happening in the sample; i.e., it corresponds to a standard DSC curve. This total signal is split by the Proteus® software into the reversing DSC signal, which is related to the heat capacity of the sample, and the non-reversing DSC signal.
The release of water as well as the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of the sample are irreversible processes and are detected in the non-reversing DSC curve. The reversing signal contains only the information about the glass transition of Eudragit®. This separation of the glass transition from all other effects allows for its accurate evaluation. Its onset was detected at 111°C; this temperature is typical for Eudragit® L100-55 (see comparison values of glass temperature onsets in [1]).
Conclusion
Just a single modulated heating with the DSC 204 F1 Nevio allows for reliable determination of the glass transition, and thus characterization of the sample. The unidentified Eudragit® was recognized as the product L100-55 by means of itsGlass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery. glass transition temperature of 111°C.
Measurements with the DSC 204 F1 Nevio are of value in the quality control of pharmaceutical substances: They ensure that the correct ingredients or excipients are used.