Introduction
Kaolin, a fine-grained, white rock, is mainly used in the ceramics, paper and polymer industries. Kaolin is an important raw material in the production of porcelain. It is also employed as a filler and coating material in paper production and as a filler for polymers such as PE-HD or rubber compounds. [1]
Especially during the SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. sintering process for porcelain, temperatures of more than 1000°C can occur, to which the raw materials and thus also kaolinite are exposed. Thermogravimetric analysis is suited for investigating the behavior of kaolinite at higher temperatures. It allows for degradation and transformation reactions in kaolin to be observed as a function of temperature.
In the following, the temperature-dependent mass change of kaolinite is investigated by means of thermogravimetric analysis.
Methods and Sample Preparation
The NETZSCH TG Libra® was employed for the thermogravimetric measurements, which were carried out under the measurement conditions detailed in table 1.
Table 1: Measurement conditions for the thermogravimetry investigation of kaolini
| Sample | Kaolin |
| Sample weight | 39.83 mg |
| Crucible material | Platinum, open |
| Temperature range | 40°C to 1100°C |
| Heating rate | 10 K/min |
| Atmosphere | Nitrogen |
Measurement Results and Discussion
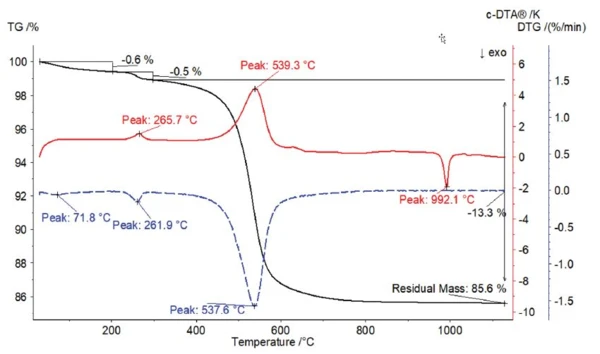
The measured TGA curve, the corresponding rate of mass change (DTG) and the calculated c-DTA® signal are depicted in figure 1. At the beginning, a mass loss of 0.6% can be observed by 200°C, which correlates with the course of the DTG curve in this temperature range with a peak at 71.8°C. This is probably due to the release of adsorbed moisture.
Upon further heating, two endothermal c-DTA® peaks can be observed at 265.7°C and 539.3°C. Both peaks are in correlation with a mass loss. Between 200°C and 300°C, a mass loss of 0.5% and the corresponding peak of the rate of mass change at 261.9°C occur. This is probably due to the release of water of CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization. With increasing temperature, endothermal dehydroxylation of the interlayers ultimately occurs resulting in the clearly recognizable mass loss of 13.3% and a sharp DTG peak at 537.6°C.
At 992.1°C, a sharp ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal c-DTA®® peak can be seen. The ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal reaction does not correlate with a mass change; i.e., it is a Phase TransitionsThe term phase transition (or phase change) is most commonly used to describe transitions between the solid, liquid and gaseous states.phase transition. This transition is irreversible and indicates the formation of mullite.
Summary
Kaolin was investigated by means of thermogravimetric analysis. With the help of the measured TGA and c-DTA® curves, various reactions of kaolin known from literature, can be identified at differente temperatures. In particular, the dehydration of kaolin can be observed very well in the thermogravimetric analysis due to the mass loss. The transition into mullite at higher temperatures is also visible if the c-DTA® signal is taken into account.