Introduction
Zirconium dioxide (ZrO2) is one of the most often studied ceramic materials. Upon heating, zirconia undergoes disruptive phase changes. By adding small percentages of yttria, these phase changes are eliminated, and the resulting material has superior thermal, mechanical and electrical properties.

Measurement Conditions and Results
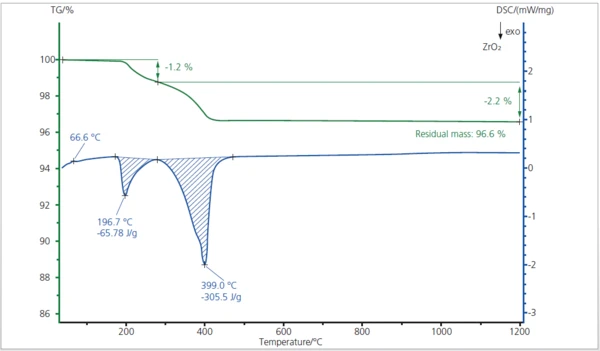
This STA measurement between room temperature and 1200°C exhibits two small losses up to 450°C (3.4% in total; green TGA curve) which correspond very well with the two ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic peaks at 197°C and 399°C in the DSC curve (blue). These effects (mass loss up to 500°C, ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic peaks with high enthalpies) can be typically observed during the binder burnout of ceramic materials. The small EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic DSC peak at around 67°C is caused by the melting of the binder.