Introduction
When comparing the measurement function of an analytical balance with that of a thermobalance, two basic differenes can be observed. When analytical balances are used for sample preparation in a laboratory, sealable panels ensure that no air draft can disturb the weighing signal; in addition, the weighing process generally takes no longer than 10 to 30 seconds. With thermobalances, on the other hand, the sample chamber is continuously purged with a carrier gas flow; and a measurement, such as one from room temperature to 1100°C at a heating rate of 10 K/min, takes nearly two hours. In the case of thermobalances, therefore, the demands placed on resistance to interference, and particularly on the long-term stability of the measuring signal, are significantly higher.
With any analytical method, the measuring instrument is adjusted and calibrated prior to investigating the sample. Then, a so-called “blank value“ is often established, which encompasses any influences which cannot be attributed to the sample. The measuring and evaluation software typically allows for correction of the measured values using the blank value. This in turn allows for the determination and elimination of systematic deviations as well as influences stemming from either the measuring instrument itself or from the selected measurement conditions.
Blank Value Determination with the Help of Correction Measurements
For thermobalances as well, the measuring signal is corrected using a blank value. Normally, this value is determined using an empty crucible and measurement conditions identical to those which will be used on the sample. This correction measurement will be saved in the software as an independent data set. Following a sample measurement, the operator can then compare the non-corrected result to the one which has been corrected as a function of temperature – all at the touch of a button in the evaluation software. However, when carrying out such a blank value determination, the greatest influences to be corrected on the measuring signal do not actually stem from the measuring instrument itself, but are attributable rather to the measurement conditions. The permanent purge gas flow and the temperature change in the sample chamber are responsible for the temperature-dependent change in flow conditions as well as for the density of the purge gas. There is therefore a change in the buoyancy undergone by the sample holder and thus also by the sample itself.
A good thermobalance is characterized by good reproducibility of the measurement results. This attests to stable measurement conditions which always register the above described, purely physical influences on the measurement result in a consistent way, and thus ensures good correction of the sample results.
Shown in figure 1 is a comparison of two blank value determinations (red and green) which attest to the good reproducibility of the TG 209 F1 Libra®. Subtraction of these blind values results in an almost ideal zero value (blue) across the entire temperature range. During thermogravimetric measurements, the sample atmosphere is often changed from an inert gas flow (usually nitrogen) to oxidzing conditions (usually synthetic air or oxygen) in order to follow up on the PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis with a targeted combustion, such as combustion of the Pyrolytic CarbonPyrolytic carbon is carbon which is generated by the pyrolysis of organic matter in an oxygen-free atmosphere. pyrolytic carbon black. Such a change in gas and the associated change in gas flow constitute a major disturbance for the weighing signal. Even a disturbance of this magnitude can be almost entirely compensated for within the correction, thanks to the mass flow conntrollers (MFC) and the associated good reproducibility of the changes in measurement conditions. The measurement uncertainty during the gas change is 0.007 mg at 600°C, which – for a very typical sample mass of 10 mg – amounts to a measurement uncertainty of ± 0.07%.
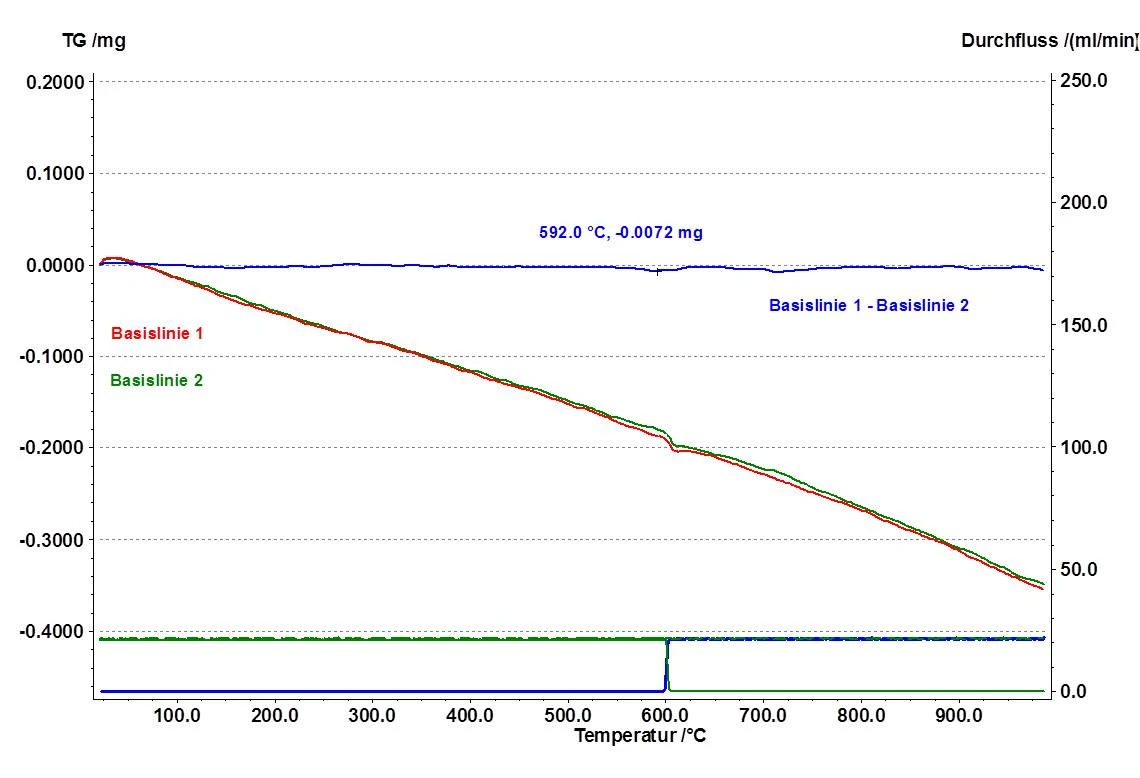
The blank value determination and the resulting ability to correct measurement values allows for the obtainment of very precise measurement results – even when the sample masses are as small as 10 mg and the pysical conditions are as described above.
Correction by Means of BeFlat®
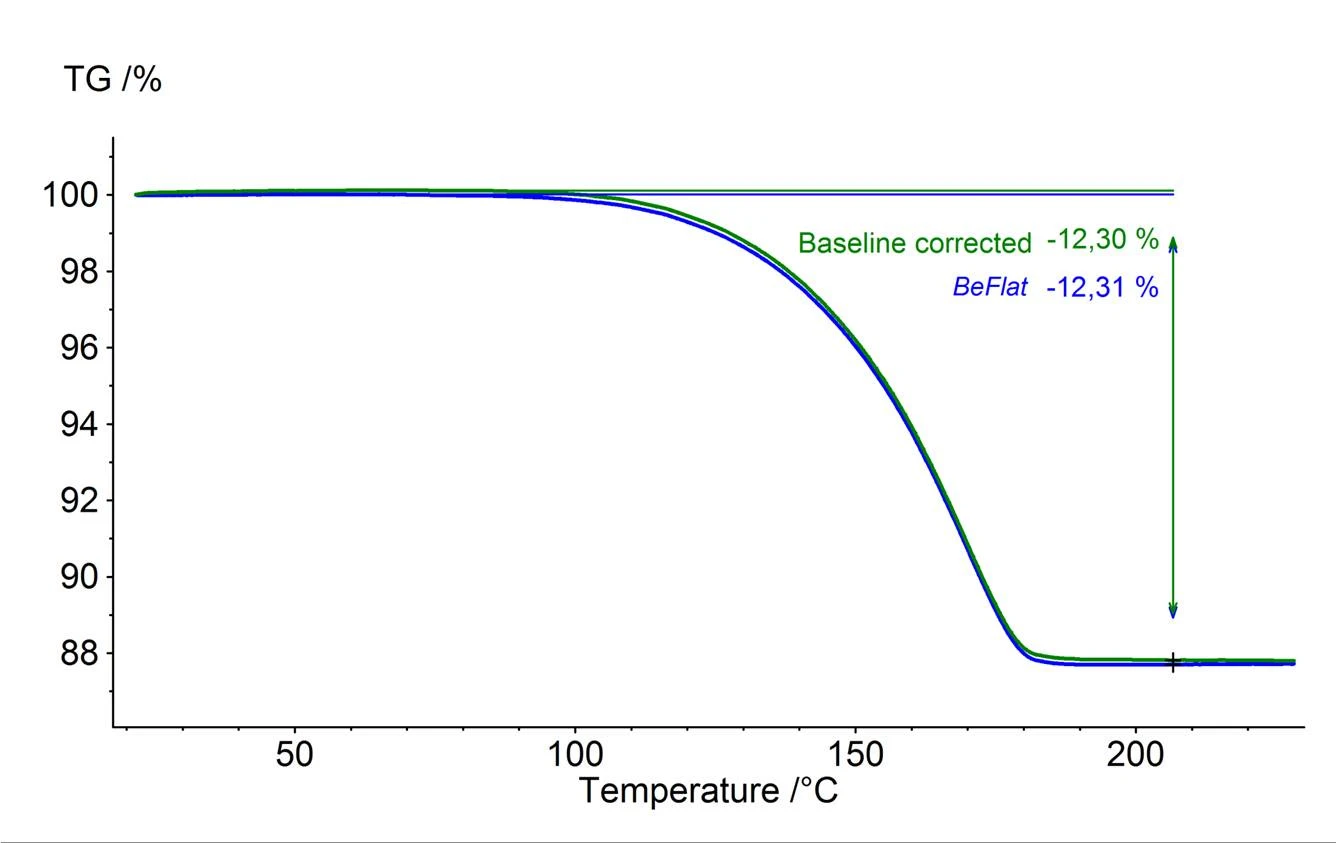
Although the above-described method for determining blank values and performing the subsequent correction does work very well, it also requires an increase in measuring effort. This is because variations in the measurement conditions – such as the crucible material and shape, type of purge gas, purge gas rate and heating rate – affect the measurement results to varying degrees. Previously, it had only been possible to correct for this by carrying out the correction measurements under exactly the corresponding changing measurement conditions for each respective measurement series.
The BeFlat® correction keeps a record of the temperature dependence for the measuring influences, the heating rate, the different purge gases (such as argon, air and nitrogen) and the gasflow rates, and can therefore provide the appropriate correction for the selected measurement conditions without having to carry out a blank value determination in the form of a correction measurement. For approximately 98% of all possible combinations of measuring influences, the corresponding temperature dependent correction is thus already available and be retrieved at any time. Of course, this correction can also be activated or deactivated via the evaluation software; the data set for the actual sample measurement therby remains unchanged.
Figure 2 shows the difference between two measurements which were carried out with empty crucibles under identical measurement conditions; one with the BeFlat® correction (blue) and the other without the BeFlat® correction (red).
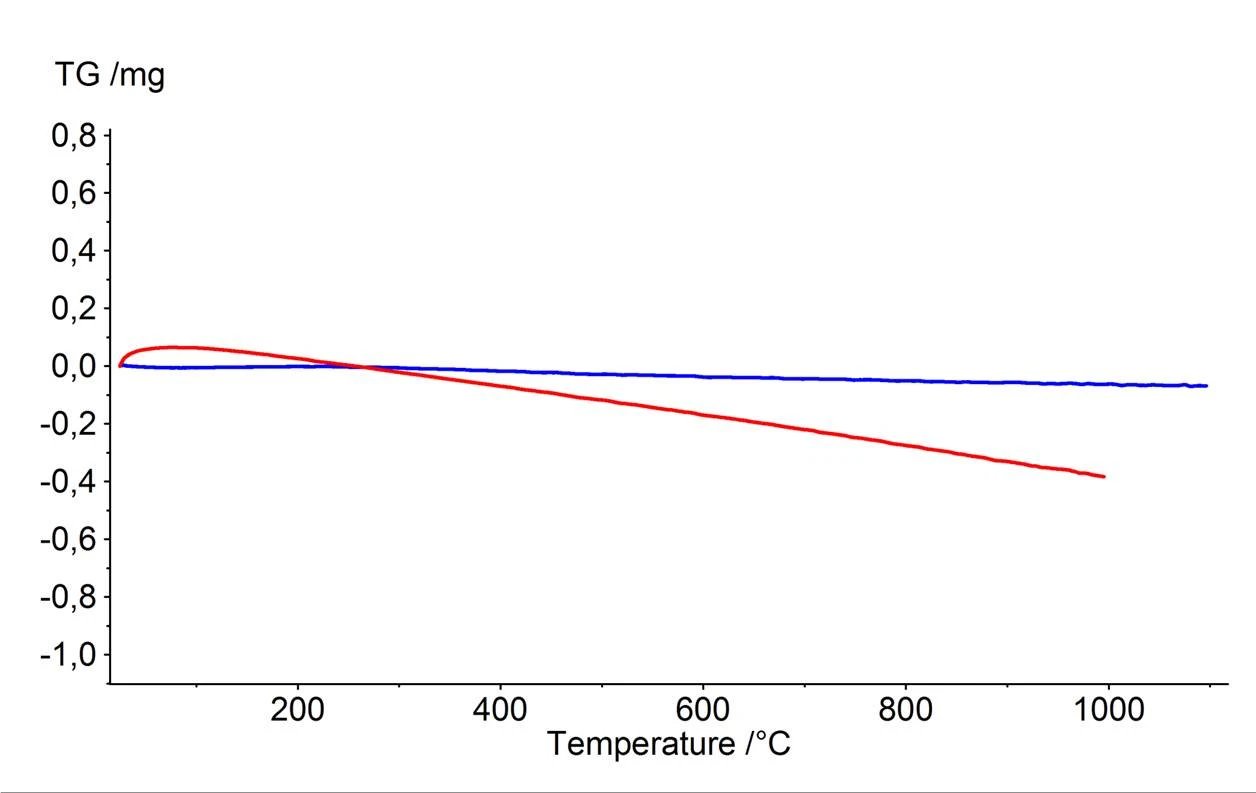
Presented in figure 3 is an example for applying the BeFlat® correction to the investigation of a thermal dehydration reaction. It can here be seen clearly that the BeFlat® correction (blue) is in very good agreement with the result of a conventional correction carried out by means of a correction measurement (green). In cases where the quality of the corrections is about the same, the advantage to using the BeFlat® correction is a huge savings in time afforded by eliminating additional correction measurements.