Tips & Tricks
What is the Influence of Crucible Lids on the Thermal Behavior of Hydrates?
Hydrates are a group of crystalline solids which release their crystal water when heated.
The release temperature is dependent, for one, on the binding energy with which the water is bound into the structure. This provides the opportunity to obtain information on the bonding relationships in the crystal structure by means of thermal analysis. The release temperature, however, is also influenced by the crucible system used. It is to be expected that the diameter of the hole in the crucible lid will influence the release temperature. Effects in the DSC or TGA curves which can be traced back to the same release of water from a hydrate can therefore vary widely in terms of their temperature position, based only upon the measurement conditions selected.

With the following example of amoxicillin trihydrate, it will be shown which effects come into play for DSC curves. Amoxicillin is an antibiotic employed for humans and animals for the treatment of bacterial infections, such as middle ear infections, bronchitis or skin diseases. The substance is not attacked by stomach acid and can therefore be administered not only as an infusion or injection but also as a tablet or suspension. Oral pharmaceutical forms mostly contain amoxicillin in the trihydrate form, a white crystalline powder [1]
Pre-Investigations by Means of TGA-FT-IR
In order to verify that the release of water by amoxicillin is not overlapped by Reacción de DecomposiciónA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of the substance, a thermogravimetric measurement in combination with a Bruker FT-IR was first carried out, the result of which is depicted in figure 1.
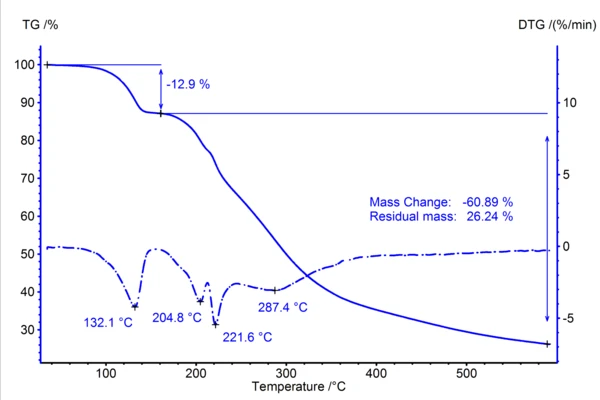
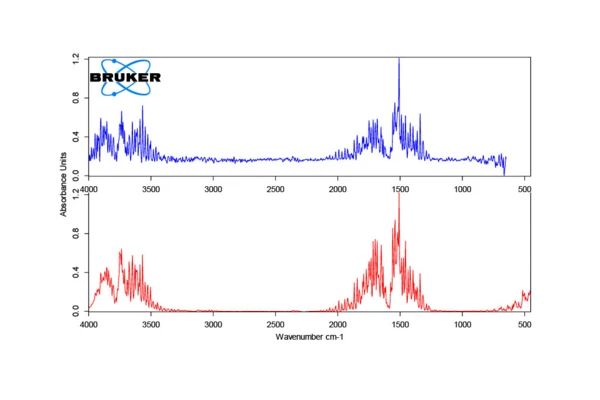
The sample exhibits several mass changes during heating to 600°C. The first step with a DTG peak temperature of 132°C corresponds to a mass loss of 12.9%. This value is in good agreement with the theoretical mass loss of 12.88%, which can be calculated based on the molar mass of amoxicillin trihydrate at 419 g/mol and the release of all three water molecules. Figure 2 confirms that the gas released is indeed exclusively water
DSC Investigation with Differently Pierced Crucible Lids
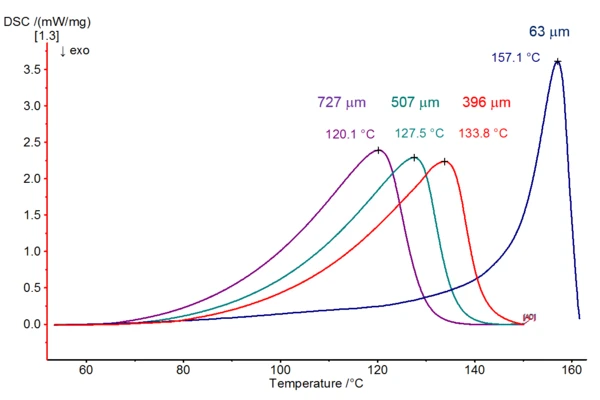
Samples with a mass of 2.25 mg each were weighed into Concavus® aluminum crucibles and the crucibles were sealed with differently pierced lids. Pictures of the prepared holes are presented below. The water release appears in the DSC curves as an endothermic peak. As can clearly be seen in figure 3, both the start of the peak and, in particular, the top of each corresponding peak are shifted to higher temperatures as the hole size decreases. A reduction of the hole diameter by a factor of 11.5 (from approx. 727 µm to approx. 63 µm) results in a shift in the peak temperature of 37 K. Additionally, the form of the peak changes: the smaller the hole in the lid, the narrower and steeper the peak.
Summary
When aluminum crucibles with pierced lids are used for the testing of organic and inorganic hydrates, the size of the hole has a significant influence on the temperature position of the resulting endothermal evaporation peak. Good reproducibility of the DSC measurement results can only be achieved when the identical measurement conditions employed also include identical hole size. Likewise, similar behavior is to be expected for all reactions in which gases are released, such as combustion or Reacción de DecomposiciónA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition reactions.