Introduction
Molybdenum has been available as a specific heat standard from NIST [1] for several decades, although not much information is available on the properties such as thermal expansion, Diffusività TermicaThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity and Conduttività TermicaThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity. According to literature [1, 2, 3, 4], pure molybdenum should not show any phase changes up to the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point. This is, however, critical because it is sensitive to oxygen at elevated temperatures. Due to the high vapor pressure of the molybdenum oxides, the material does generally not change properties because of surface OxidationOxidation can describe different processes in the context of thermal analysis.oxidation. The formed oxides simply evaporate from the surface. All these special properties of molybdenum make it a reasonable substance for a multi-property standard material.
Experimental
Measurement of different thermophysical properties like thermal expansion, density change, specific heat and thermal diffusivity were carried out on a pure (99.99%) molybdenum material. Pushrod dilatometry (DIL) was employed for measurement of the thermal expansion and determination of the density change. Differential scanning calorimetry (DSC) was used to measure the specific heat. The thermal diffusivity was determined using the laser flash technique (LFA). The test results allow a detailed insight into the material’s behavior under thermal treatment and it was also possible to determine the thermal conductivity. A comparison of all test results with available literature data was made.
Tests were carried out on different samples prepared from the original block and measured between -125°C and 1400°C. Therefore, it was possible to evaluate this material as a possible candidate for a standard material for different thermophysical properties over a broad temperature range.
The pure molybdenum (99.99%) was supplied by Plansee SE, Reutte, Austria. A large block, 30 mm in diameter and 120 mm in length was used for the analysis. From the cylinder block, different samples were prepared for the various test techniques. For each measurement method, two samples were prepared and tested two to three times. The Thermal StabilityA material is thermally stable if it does not decompose under the influence of temperature. One way to determine the thermal stability of a substance is to use a TGA (thermogravimetric analyzer). thermal stability and homogeneity of the material were checked and the repeatability of the test results was determined.
Test Results
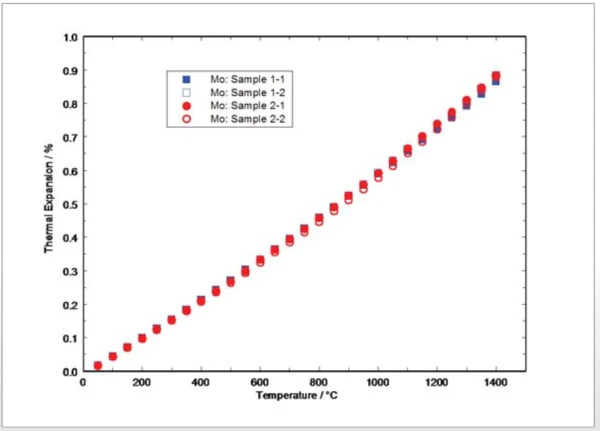
Presented in figure 1 are the measured thermal expansion results for the two different molybdenum samples measured twice. Data scattering between the samples and the different experiments are generally within ±1.5%. Considering the accuracy and repeatability of the instrument employed, the influences of surface effects and the impact of the evaporation of oxides, scattering of the data is in an acceptable range. The results give no indication of material inhomogeneities or changes in the thermal expansion values between the different heating runs.
Depicted in figure 2 are the Volumetric ExpansionThe volume of a gas, solid or liquid changes if the temperature, the pressure or the forces acting on that gas/solid/liquid change. In the case of thermal analysis, we are looking at temperature-dependent changes.volumetric expansion and the density change of molybdenum versus temperature. The Volumetric ExpansionThe volume of a gas, solid or liquid changes if the temperature, the pressure or the forces acting on that gas/solid/liquid change. In the case of thermal analysis, we are looking at temperature-dependent changes.volumetric expansion was determined from the measured thermal expansion assuming an isotropic behavior of the material and therefore, the same expansion behavior in all directions. Density calculation was based on the Volumetric ExpansionThe volume of a gas, solid or liquid changes if the temperature, the pressure or the forces acting on that gas/solid/liquid change. In the case of thermal analysis, we are looking at temperature-dependent changes.volumetric expansion and the room-temperature bulk density of 10.216 g.cm-3. The room-temperature bulk density was determined from the originally supplied sample block by measuring mass and volume.
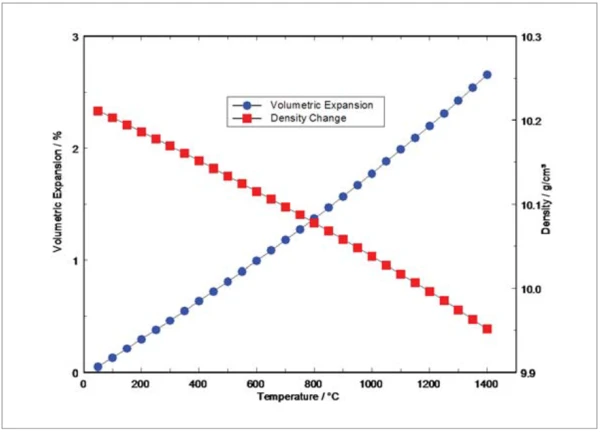
Figure 3 shows the specific heat values measured with the differential scanning calorimeter. Again, both samples were measured twice in the low-temperature steel furnace (-125°C to 300°C) and in the high-temperature platinum furnace (300°C to 1275°C). The deviation between the individual results was within ±2.0% and therefore by far within the stated uncertainty of the instrument employed for the tests. The values show a strong increase versus temperature in the low-temperature range. This behavior can be expected according to the well-known Debye theory [5]. At high temperatures, the values increase nearly linear. This is in perfect agreement with the solid state physics (Rule of Dulong and Petit, [5]). No overlapping transition or other thermal effects were detected within this temperature range, clearly indicating that no phase change occurs in the material between -125°C and 1275°C. This fulfills the condition as a standard material since no structural changes in the temperature range of interest occur.
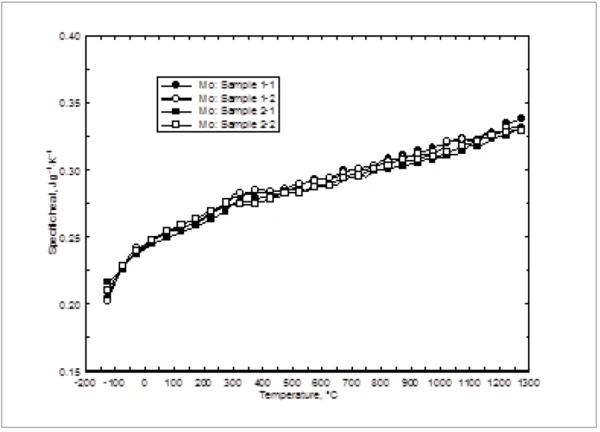
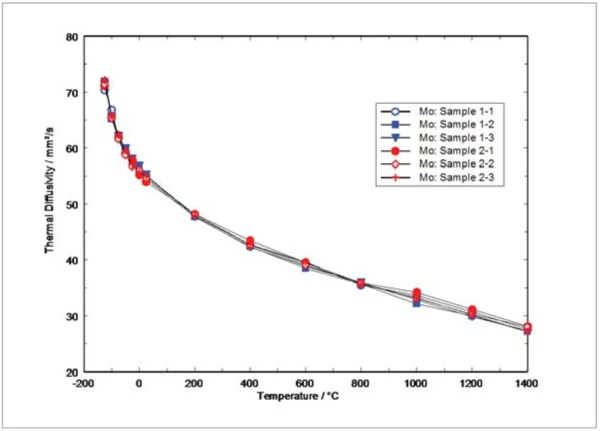
Figure 4 shows the measurement results for the thermal diffusivity gathered from the different flash devices used for the tests. It can clearly be seen that the thermal diffusivity decreases versus temperature. The decrease follows the T-1 behavior below 600°C resulting in a nearly linear decrease at higher temperatures. Such behavior is typical for predominately phonon-conducting materials such as ceramics or graphite materials. Therefore, it might be the case that the electron contribution to the heat transfer is small for this metallic material. Scattering of the measurement results from run to run and from sample to sample and is generally within ±2%. Only at 1000°C, slightly higher scattering (±3%) was obtained. A possible explanation for this might be the evaporation of molybdenum oxides in this temperature range influencing the samples emissivity and therefore the absorption of laser light and emission of infrared light.
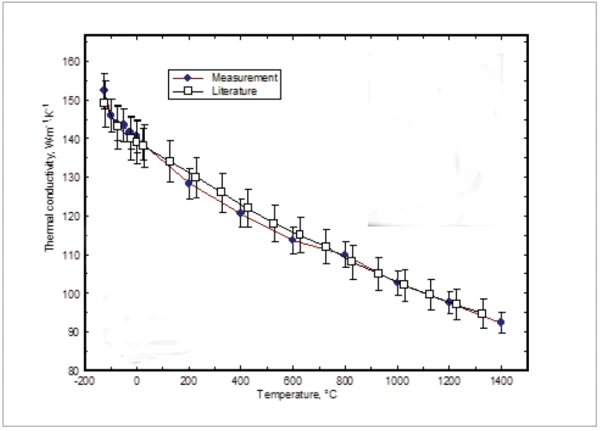
Presented in figure 5 are the results of the thermal conductivity determined by multiplying the measured density, specific heat and thermal diffusivity. The density data below room temperature and the specific heat above 1275°C were determined by a linear extrapolation of the measured data. It can clearly be seen, that the thermal conductivity follows the temperature dependence of the thermal diffusivity. A comparison with literature values [6] was also made. Assuming 5% accuracy of the literature values and 3% uncertainty of values based on the measurement, the results are in very good agreement.
Conclusion
Various thermophysical properties (thermal expansion, density change, specific heat, thermal diffusivity, thermal conductivity) were measured on high-purity molybdenum. The comparison with literature values indicated the quality of the measurement results and the reliability of the material. It can be assumed from the tests results that pure molybdenum might be a reasonable candidate to be used as a standard material up to high temperatures above 1200°C. It may be used as a calibration standard for various thermophysical properties. Further tests at various different laboratories and test institutes would be appreciated to prove the capability of the material.