Introduction
The research on innovative battery materials is currently a vibrant field, driven by the necessity of finding alternative or complementary solutions to the current dominant technology, i.e., lithium-ion batteries [1]. Since this technology has limitations in terms of sustainability, availability of raw materials, and energy/power performances, a variety of newly developed materials for the cathode, anode and electrolyte that tackle these challenges are continuously being proposed. Thermoanalytical techniques can do well in supporting electrochemical energy storage research, as already demonstrated in previous application notes. Until now, we have focused on presenting examples about standard lithium-ion battery technology. [2, 3, 4]
In this application note, we will show how these techniques can also support the study of novel materials for batteries. Specifically, thermogravimetric analysis simultaneously coupled with a mass spectrometer (TG-MS) and Fourier transform infrared spectroscopy (TG-FT-IR) was performed on samples of molybdenum trioxide (MoO3) modified by the insertion, in the spaces within its crystal structure, of octylamine, an organic molecule, with a molar ratio of MoO3:octylamine of 1:1 [5]. The octylamine is inserted to provide a source of carbon that is in intimate contact with the MoO3 (figure 1).
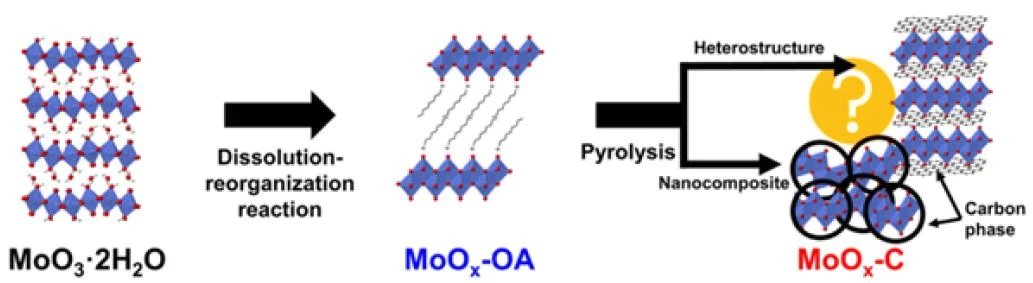
This inorganic material is meant to be used as a cathode material, and the carbon acts as a promoter of the electrochemical reactions by enhancing the conduction of electrons. Hence, the carbon is beneficial for achieving high performance with layered oxides such as MoO3, which are often semiconductors or insulators. After the insertion of the organic molecule, the modified material (MoOx-OA) is subject to a PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis process, and the use of TG-MS and TG-FT-IR was necessary to investigate which changes occur in the material upon this treatment. In particular, the objective is to understand whether carbon is formed during the PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis, and whether this carbon formation affects the molybdenum oxide structure.
Measurement Conditions
TG-MS and TG-FT-IR analyses were conducted employing a NETZSCH TG 209 F1 Libra® thermal analyzer, operating under argon flow at a heating rate of 10 K/min. The temperature range spanned from 40°C to 70°C in open Al2O3 crucibles containing approximately 20 mg of the sample Mass spectrometric (MS) data were collected using a QMS 403 Aëolos® Quadro mass spectrometer within the 10 - 300 m/z range. Additionally, Fourier-transform infrared (FT-IR) spectra were obtained using a BRUKER Invenio spectrometer in absorption mode, covering the range of 4500 to 650 cm−1 at a resolution of 4 cm−1.
Measurement Results
The findings suggest that MoOx-OA experiences three significant structural transitions during PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis. These transitions can be comprehensively elucidated by analyzing the evolving gaseous products through TG-MS and TG-FT-IR at various temperatures.
In a first stage between 120°C and 200°C (in yellow in figure 2), the thermogravimetric results indicate a twostep mass loss of approximately 24 wt%, coinciding with the release of gaseous species. The m/z = 17 and 18 signals in the TG-MS results imply the desorption of surface water molecules and ammonia (NH3), possibly originating from octylamine Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition. The m/z = 30 peaks correspond to the ion [CH2NH2]+, indicative of octylamine ionization. Additionally, m/z = 28 can be attributed to hydrocarbons, CO2, or N2, and m/z = 44 to hydrocarbons or CO2. The TG-FT-IR results in figure 3 support the evolution of molecular octylamine and water, along with traces of CO2 and NH3 in this temperature range (see also figure 4a). Therefore, the primary causes of initial interlayer shrinking are the loss of loosely bonded octylamine and water through evaporation, along with the initial onset of octylamine Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition.
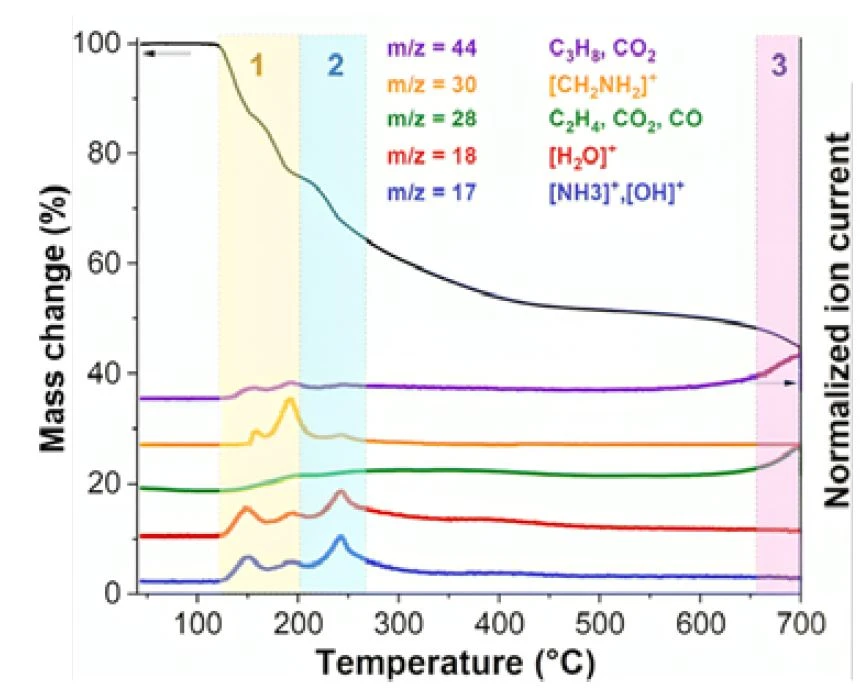


A second stage up to 350°C (in light blue in figure 2) is characterized by an accumulated mass loss of approximately 43 wt%, detected by TG and accompanied by simultaneous MS signals at m/z = 17, 18, and 44. This indicates further release of water and octylamine Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition products (NH3 and hydrocarbon fragments). FT-IR spectra in the 3000 - 2800 cm−1 range confirm the evolution of hydrocarbons, while the ambiguous pattern in the 1500 - 650 cm−1 region prevents assignment to a specific molecule (figure 4b). Strong ammonia absorption patterns in the same temperature range confirm octylamine Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition.
In the final stage (in violet in figure 2), a mass loss is observed above approximately 650°C, with a cumulative mass loss of 58 wt%. This corresponds to an MS signal at m/z = 44, attributed to CO2, indicating carbothermic reduction of MoO3 to MoO2 caused by the carbon left as product from the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of the octylamine. Another strong peak at m/z = 28 can be assigned to both CO2 and CO, and FT-IR spectra at this temperature confirm the simultaneous presence of these two gases (figure 3 and 4c).
Conclusion
In summary, it was observed that during the heating process, certain portions of the loosely bound molecular octylamine and its Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition products are released from the interlayer space before undergoing conversion into elemental carbon. Additionally, a pronounced carbothermic reduction of the oxide occurs above 650°C; this modifies the structure of the molybdenum oxide by removing oxygen from its structure. The production of carbon after PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis was confirmed, but the evaporation/ Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of a part of the octylamine removed a substantial part of this source of carbon. Therefore, future efforts aimed at enhancing the synthesis route can prioritize the utilization of more strongly bonded and/or less volatile organic molecules, since an increased amount of carbon can improve the electrochemical performance of the battery cathode material. Nevertheless, the resulting material after PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis fared better as battery cathode than the MoO3 reference sample in terms of the capacity reached at high currents and the stability of the battery itself.
The combination of TG-MS and TG-FT-IR was necessary in order to identify and/or confirm the formation of certain gases in the various steps of the PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis reaction.