Introduction
Magnesium stearate is widely used as a lubricant in the production of cosmetics and pharmaceuticals. It is commercially available as a mix of several fatty acid salts that may vary in proportion. Furthermore, magnesium stearate can be found as monohydrate, dihydrate and trihydrate. In fact, the physical properties, and particularly the lubricating properties of this material are influenced by its moisture content and its hydration state. For these reasons, the properties of magnesium stearate can vary considerably from one manufacturer to another [2, 3].
The varying properties of magnesium stearate can be investigated by means of DSC, which is a particularly easy and fast method for obtaining a fingerprint of the substance. Another method of thermal analysis, TGA, can help give an indication about the hydration state of magnesium stearate.
In the following, a magnesium stearate sample was characterized by means of DSC and TGA measurements. In addition, the influence on the thermal properties resulting from a 2-hour storage period at 60°C and 120°C in a dry nitrogen atmosphere was studied.
Test Conditions
The measurements were carried out with a DSC 214 Polyma and a TG 209 Libra® in a dynamic nitrogen atmosphere. Sealed Concavus® crucibles with pierced lid were used.
Test Results
Figure 2 depicts the TGA curve of magnesium stearate. Between room temperature and 125°C, the sample loses 3.5% of its initial mass, resulting from the release of water. In this temperature range, two peaks are detected in the DTG-curve (1st derivative of the TGA curve) indicating two steps: first, the surface water evaporates (1st mass-loss step 1.1 %), then the hydrate water is released (2nd mass-loss step 2.4 %) A molar mass of 591.27 g/mol for magnesium stearate results in a theoretical loss of water of 2.95% for the monohydrate form, 5.74% for the dihydrate form and 8.37% for the trihydrate form. Therefore, the detected mass loss is an indication for the monohydrate form of magnesium stearate: During heating, the sample first loses its surface water (1st mass-loss step 1.1.%) before the crystal water is released.
This result can be confirmed by the TGA curves described by D. Lugge in [4]: For a pure magnesium stearate dihydrate and pure trihydrate, the mass loss would be at a lower temperature.
Therefore, the measured sample contains at least the monohydrate form of magnesium stearate. The difference between the theoretical loss of water of 2.95% and the measured one of 3.5% between room temperature and 125°C may come from the evaporation of surface water and/or from the presence of dihydrate/trihydrate in the specimen.
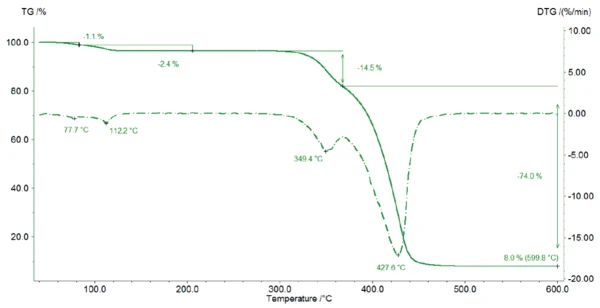
Figure 3 shows the DSC curve of magnesium stearate during heating to 200°C. The peaks detected at 78.3°C, 91.8°C, 95.8°C and 116.0°C are partly due to the release of surface water and bound water, as indicated by TGA. The evaporation process is probably overlapped by melting of the sample components.
The endothermal effect at 145.2°C is not associated with a mass loss and is probably due to melting of a constituent of the sample.
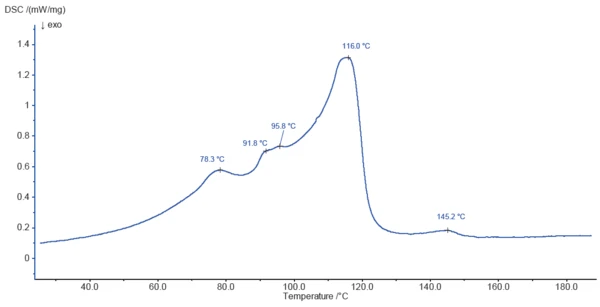
Figure 4 displays the DSC curves of magnesium stearate as received along with the DSC curves of the material after storage for 2 hours at 60°C (red curve) and at 120°C (black curve), respectively.
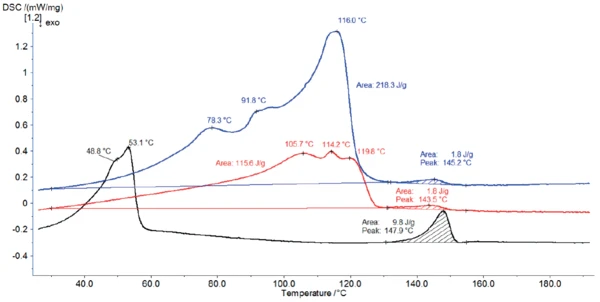
The storage at 120°C (black curve) changed the DSC profile completely: It removed the hydrate water. According to Ertel and Carstensens [5], heating at 105°C not only removes water but also changes the structure of the crystals. Here, the storage at 120°C yielded a structure with peak temperatures at 49°C and 53°C.
During storage at 60°C, the sample loses part of its water. Therefore, the amount of water released during heating in the DSC decreases, lowering the peak enthalpy between 30°C and 130°C. Additionally, the effects are slightly shifted to higher temperatures.
The peak between 130°C and 155°C is detected for all three measurements and is in quite good agreement with the theoretical melting range of pharmaceutical grade magnesium stearate (130°C to 145°C [6]). However, its enthalpy is much higher for the sample stored at 120°C. As mentioned above, this higher peak enthalpy after storage at 120°C is probably associated with a change in the structure of magnesium stearate [5].
Conclusion
Storage of magnesium stearate at different temperatures yields differing thermal behavior, which can be seen in the change of the DSC curves. Thermal treatment causes the release of differently bound water, the water release temperature giving an indication of the type of water (e.g., surface water). The differences in the DSC curves probably also result from changes in the crystal structure of the sample during storage.
Commercially available magnesium stearates are mixtures of different fatty acids that can vary from one manufacturer to another, making TGA and DSC indispensable tools for verification prior to preparing a pharmaceutical composition.