Introduction
Sorbitol is a sugar alcohol found in fruits that is frequently applied as a sweetener in food products. It exists in four anhydrous crystalline phases plus the hydrate. This PolymorphismPolymorphism is the ability of a solid material to form different crystalline structures (synonyms: forms, modifications).polymorphism has an influence on the properties of this substance: Each form behaves differently with regard to melting and to water absorption [1].
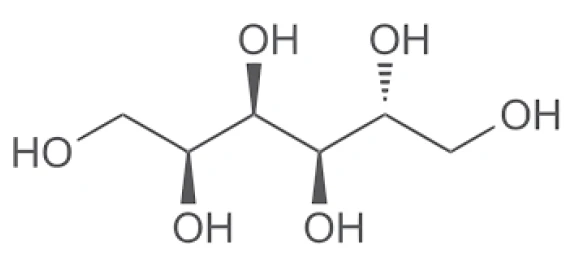
Test Conditions
A sorbitol sample (mass: 3.81 mg) from Sigma-Aldrich was prepared in a Concavus® crucible and measured with the DSC 204 F1 Nevio. An initial heating was carried out between -80°C and 150°C at a heating rate of 10 K/min. Then the sample was cooled down at 10 K/min and heated again in the same temperature range. After that, the crucible was kept for 24 hours at room temperature, before it was measured a third time between -80°C and 150°C under the same conditions. The DSC measurements were carried out in a dynamic nitrogen atmosphere.
Additionally, PXRD measurements were performed on two sample states:
- Sample as received
- Sample after heating to 150°C and 24 hours at room temperature
These measurements were carried out with the Bruker D8 Advance diffractometer at solid-chem GmbH.
Test Results
Figure 2 displays the DSC curves of sorbitol during the three heating runs. The endothermal peak with an extrapolated onset temperature of 91°C, detected during the first heating, results from the melting of the sample. This temperature is typical for the modification known as a gamma form, which is the most suitable for commercial applications because it is the most stable one.
After cooling at 10 K/min, no melting peak was detected within the subsequent second heating: The sample no longer exhibits any crystalline phase and it is in an amorphous state with the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition at -1°C (mid temperature).
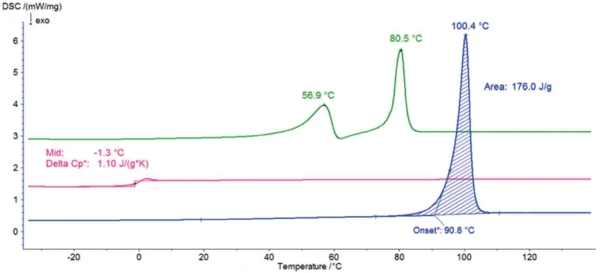
One day at room temperature is enough to allow CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization to take place. However, the peaks detected at 57°C and 81°C (peak temperatures) prove that it is a different crystalline form than the one detected during the first heating. This DSC curve is typical for the modification called crystallized melt. This form is more hygroscopic than the gamma one. However, it is used commercially because of its transparent and glassy appearance, for example, in the production of hard candies.
The melting temperatures of the crystalline forms measured in this work are compared with different literature sources in table 1.
Table 1: Peak temperatures of the crystalline forms: crystalline melt, alpha, gamma and Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition temperatures of the amorphous form for this work and different sources.
| Form/Temperature [°C] | This work | Source [1] | Source [3] | Source [4] | Source [5] |
|---|---|---|---|---|---|
| Crystallized melt (1st peak) | 56.9 | 54.5 | 55 | - | - |
| Crystallized melt (2nd peak) | 80.5 | 70.8 | 75 | - | - |
| Alpha | - | 85.9 | 86 | 88.5 | - |
| Gamma | 100.4 | 98.0 | 97 | 100 | 101.7 |
| Amorphous | -1.3 | - | - | - | -0.4 |
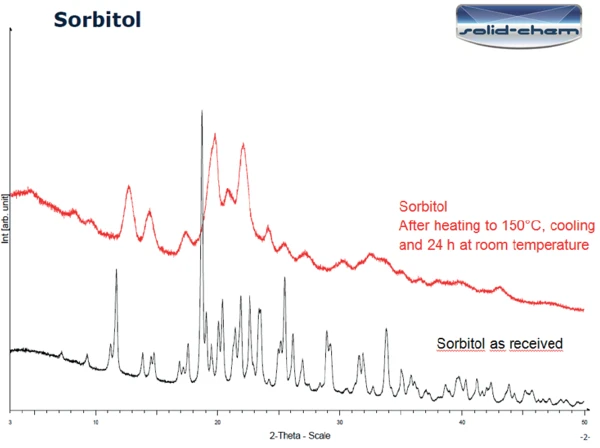
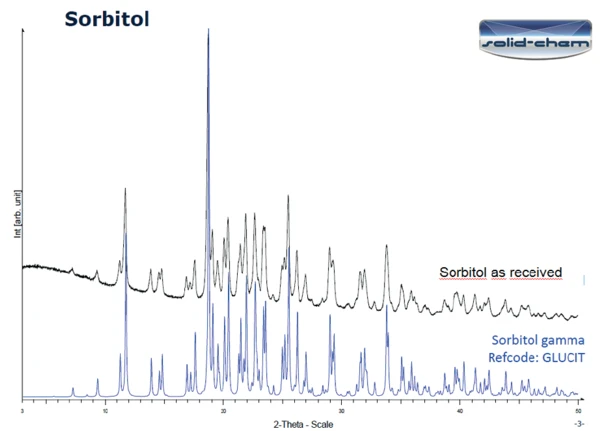
Figure 3 displays the PXRD results on the sample as received (bottom) and on the sample after heating to 150°C followed by 24 hours at room temperature (top). The two curves differ significantly. The peaks detected in the measurement of the sample as received correspond to the gamma form of sorbitol (figure 4). According to literature ([1], figure 6 [X-ray powder diffraction pattern of the sorbitol crystallized melt polymorph]), the curve after heating to 150°C and one day at room temperature can indeed be classified as the crystallized melt of sorbitol.
Conclusion
Just a single heating with the DSC 204 F1 Nevio allows for identification of the polymorphic form of the supplied sorbitol. During cooling of the gamma form at 10 K/min from the melt, sorbitol does not crystallize but forms an amorphous phase. This amorphous structure can crystallize at room temperature as a new modification called crystallized melt. These results were confirmed by PXRD measurements.
Each of the sorbitol modifications has different physical properties. That is why they have to be characterized before processing. The DSC 204 F1 Nevio provides the necessary results easily, quickly and reliably.
Acknowledgement
NETZSCH would like to thank solid-chem GmbH in Bochum, Germany, for carrying out the PXRD measurements and evaluation.