Introduction
Temperature-modulated thermogravimetric analysis refers to thermogravimetric measurements under modulated temperature conditions with the aim of determining activation energies in a direct way. In the case of a temperature- modulation TGA experiment, the temperature is the sum of an underlying linear heating rate and temperature oscillations. The amplitude of the temperature oscillations usually varies from 5 K to 10 K. This variation is much higher than in modulated DSC, where the typical temperature amplitude is about 0.5 K. The period is usually from 60 to 300 s, and the underlying heating rate from 1 K/min to 20 K/min. The main kinetic equation is
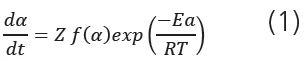
where α is the degree of conversion, t is the time, Z is the preexponential factor, Ea is the activation energy, R is the gas constant, and T is the (absolute) temperature.
Measurement on Polystyrene (PS) – Parameters andResults
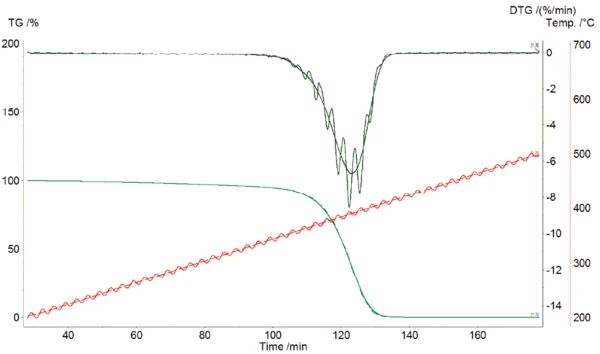
A chemical reaction runs faster at higher temperatures and slower at lower temperatures. Therefore, modulated temperature with high-temperature amplitudes results in oscillations of the reaction rate. These oscillations can be well seen in the DTG curve for the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of polystyrene, PS (figure 1).
This measurement was carried out with the TG 209 F1 Libra® at a heating rate of 2 K/min, and an amplitude of 5 K for a period of 200s. The red curves are the modulated and underlying temperature, the green curves are the modulated and underlying TGA and the black curves are the modulated and underlying DTG. The underlying curves are calculated as the average over one period.
Calculation of the Activation Energy
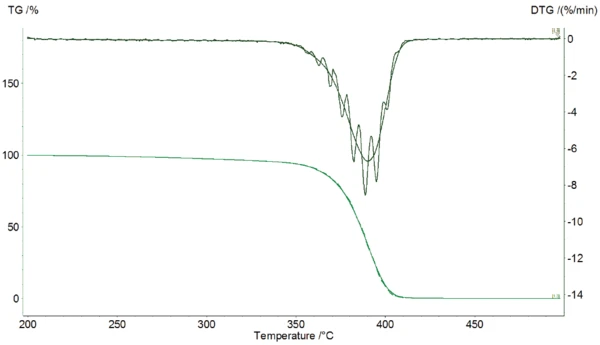
The amplitude of the DTG curve can be found by Fourier analysis which is proportional to the underlying DTG curve (see figure 3). This DTG amplitude depends on the activation energy of the chemical reaction. Therefore, the activation energy Ea can be calculated directly from the DTG amplitude ADTG, the absolute value of the underlying DTG and the temperature amplitude AT of the following equation:
Ea = ADTG/(AT * |DTGunderlying|) * R*T2 (2)
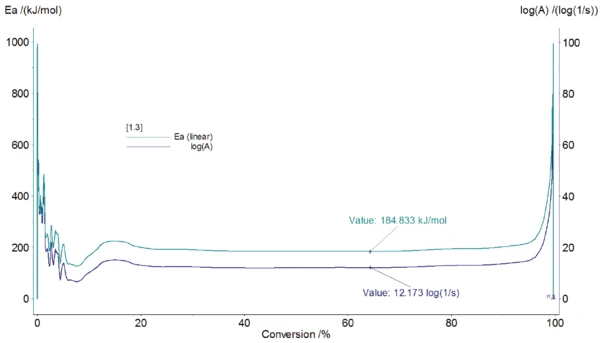
The values for the activation energy are about constant for each individual reaction step. For polystyrene, this value calculated by equation (2) is about constant for the conversions from 5% to 95% (see figure 4) where the activation energy amounts to 184.8 kJ/ mol and the pre-exponential factor to 12.17 log(1/s).
The Proteus® software allows for calculation of the activation energy in accordance with three methods: ASTM E2958 and two more accurate methods: linear and nonlinear [1].