Introduction
Phenol formaldehyde resins are thermosets obtained by the polycondensation of formaldehyde with phenol or substituted phenol. They are the first synthetic resins to have been developed. The most famous phenol formaldehyde resin, best known as Bakelite, got its name from Leo Baekeland who produced it commercially.
Test Conditions
The Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing of a phenol formaldehyde resin was measured with the DSC 214 Polyma using high-pressure crucibles. The Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing of PF is a polycondensation reaction which is connected with a loss of water. In an open crucible, the evaporation of water would cause an EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic effect in the DSC curve which superimposes the ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing reaction.
Three samples of approx. 20 mg each were prepared and measured at 2, 3 and 5 K/min from room temperature to 260°C.

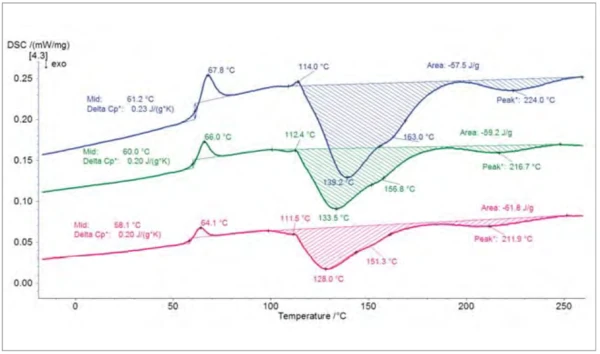
Test Results
The EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic step detected in the three DSC heating curves comes from the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition of the uncured polymer. As expected, it shifts to higher temperatures as heating rates increase (mid-point at 58°C and 61°C for the measurements at 2 K/min and 5 K/min, respectively). It is overlapped with a RelaxationWhen a constant strain is applied to a rubber compound, the force necessary to maintain that strain is not constant but decreases with time; this behavior is known as stress relaxation. The process responsible for stress relaxation can be physical or chemical, and under normal conditions, both will occur at the same time. relaxation peak that comes from the release of mechanical StressStress is defined as a level of force applied on a sample with a well-defined cross section. (Stress = force/area). Samples having a circular or rectangular cross section can be compressed or stretched. Elastic materials like rubber can be stretched up to 5 to 10 times their original length.stress within the sample. The ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic double peak between 100°C and 250°C is due to the Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing of the resin. All three curves additionally have a shoulder at temperatures ranging from 151°C (at 2 K/min) to 163°C (at 5 K/min). The Curing (Crosslinking Reactions)Literally translated, the term “crosslinking“ means “cross networking”. In the chemical context, it is used for reactions in which molecules are linked together by introducing covalent bonds and forming three-dimensional networks.curing reaction is overlapped with a small EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic peak detected at 112°C (2 and 3 K/min) and 114°C (5 K/min) that most probably comes from the melting of an additive.
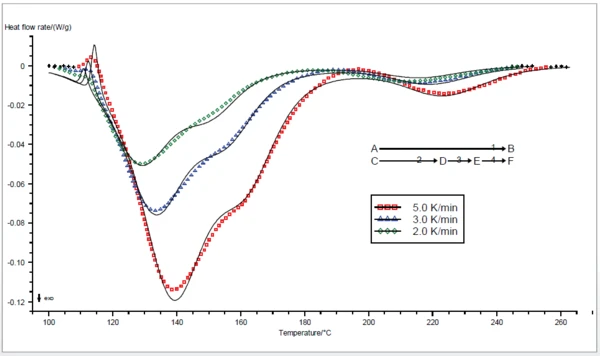
These three curves were used to determine the kinetics of the curing reaction by means of the NETZSCH Advanced Software Thermokinetics. Due to the complex peak structure, it is supposed that the curing is a three-step reaction. The melting peak was also taken into account for the kinetics model with an independent, one-step reaction.
The result is given in figure 2. The best model for the curing reaction is a three-step one in which each step is of the n-th order type with autocatalysis. Additionally the melting eff ect is taken into consideration by means of a one-step reaction of the second order. With a correlation coeffi cient of over 0.99, the curves calculated by the kinetics model (solid lines) are in good agreement with the measured ones (dotted lines), which confirms the initial assumption.
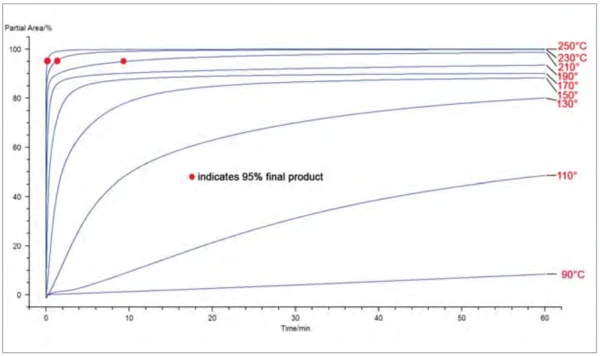
The kinetic model can now be used to predict the rate of reaction for a specifi ed temperature program. As an example, figure 3 shows the curves of the final product determined by the partial area as a function of time for different temperatures between 90°C and 250°C. It is also possible to predict the percentage of final product during any temperature program defined by the user, as shown in figure 4.
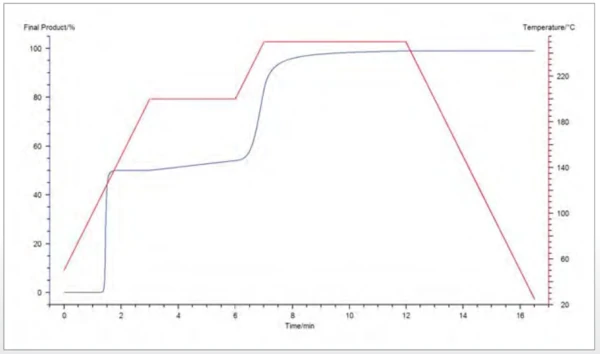
Conclusion
High-pressure crucibles were used with the DSC 214 Polyma to investigate the curing reaction in a phenol formaldehyde resin. Three measurements at diff erent heating rates allow for the determination of the reaction kinetics by means of the Thermokinetics software. The kinetics model can then be used to make predictions with regard to the behavior of the system under userdefined temperature conditions like processing conditions.