Introduction
In pharmacy, there is hardly any active ingredient about which more has been written than acetylsalicylic acid (or ASA for short; in English- and German-speaking countries, the brand name Aspirin™ is often even used as a synonym). Its success story began at the end of the 19th century when Dr. Felix Hoffmann synthesized the substance at the BAYER laboratories for the first time without impurities. Nowadays, it is still one of the most popular pharmaceuticals, used across a broad therapeutic range. It belongs to the group of non-steroidal antiinflammatory drugs (NSAIDs) and is indicated for the treatment of pain, fever and inflammation. In addition, it is used to prevent recurrence of heart attack or stroke in high-risk patients. In 1977, ASA was added as an analgesic to the “essential drug list” of the WHO (World Health Organization). [1]
This is the last of four application notes that examine in more detail the thermal behavior of acetylsalicylic acid; the first three addressed Réaction de DécompositionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition in different gas atmospheres, decomposition kinetics, and the resulting gas species [2, 3, 4].
Experimental
For the investigation of the thermal decomposition of acetylsalicylic acid, thermogravimetric measurements (TGA) were carried out with the NETZSCH TG 209 Libra® under a helium atmosphere. For supportive interpretation, the thermal analyzer was additionally coupled with a 403 Aëolos® quadrupole mass spectrometer. The exact measurement conditions are detailed in table 1.
Table 1: Measurement parameter
| Parameter | Acetylsalicylic Acid |
|---|---|
| Analyzer | TG 209 Libra® with QMS 403 Aëolos® |
| Sample holder | TGA, type S |
| Crucible | Al2O3, 85 μl, open |
| Sample mass | 8.35 mg |
| Temperature program | RT to 500°C, heating rate: 10 K/min |
| Atmosphere | Helium* (100 ml/min) |
*In this work, a helium atmosphere was in line with the measurements included in the previous Application Notes 208, 209 and 210 (Part 1 through 3).
Measurement Results and Discussion
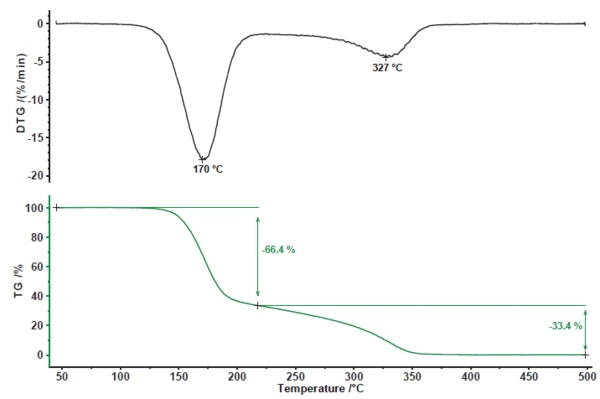
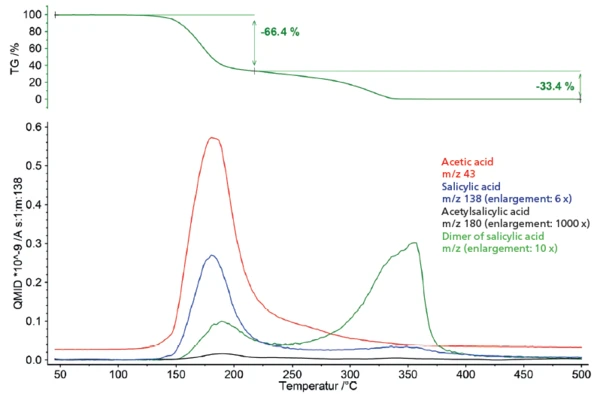
The PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis of acetylsalicylic acid exhibits two mass-loss steps (figure 1). The first mass-loss step of 66.4% is associated with a peak in the mass-loss rate (DTG) at 170°C. The second mass-loss step amounts to 33.4% with a peak in the DTG curve at 327°C.
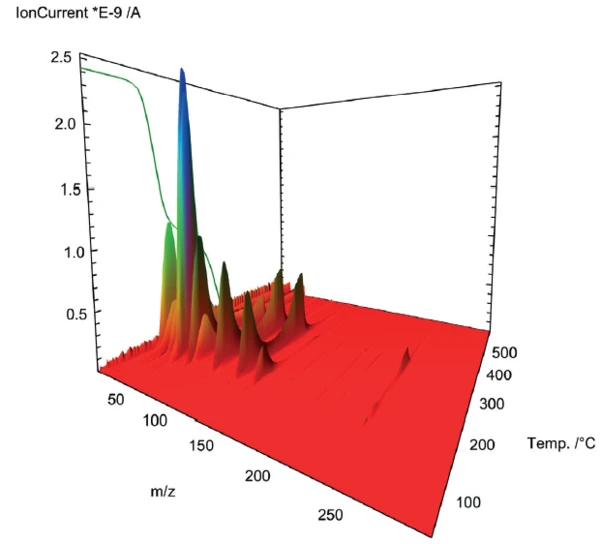
The mass spectrometer coupling used to gain deeper insight into the PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis of acetylsalicylic acid shows a complex profile for outgassing in the two mass-loss steps (figure 2). For closer examination, the mass spectra of the respective steps were extracted and subjected to a database comparison with the “NIST MS Library”.
Database analysis of the first mass-loss step shows mainly an overlapping release of acetyl acid and salicylic acid, indicating degradation of the acetyl functionality of acetylsalicylic acid (figure 3). Along with the two main outgassing products, higher mass numbers (> 138 u) are also represented in the spectrum, which can be attributed to the dimer of salicylic acid. In addition, partial evaporation of the undecomposed acetylsalicylic acid within the first mass-loss step cannot be excluded as a possibility since all main masses of the acetylsalicylic acid spectrum (43, 60, 92, 120, 138 u) are overlapped by the previously mentioned decomposition products.
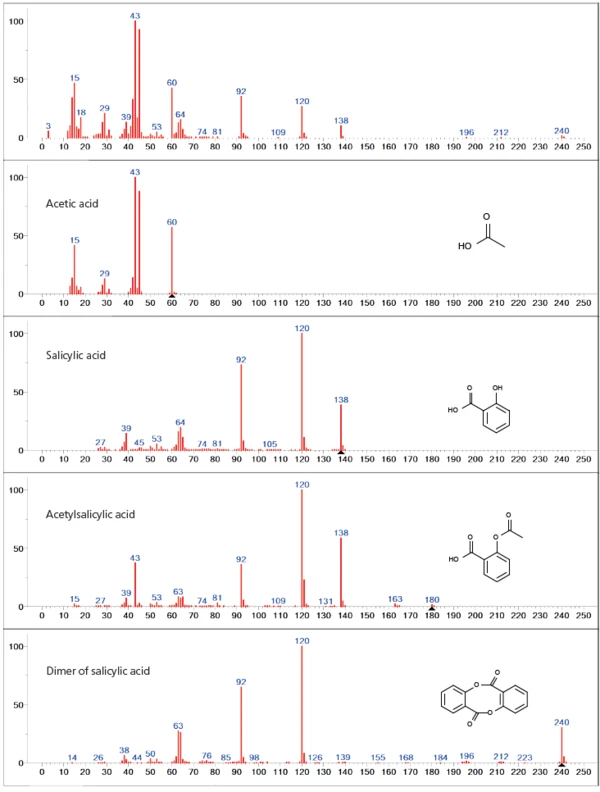
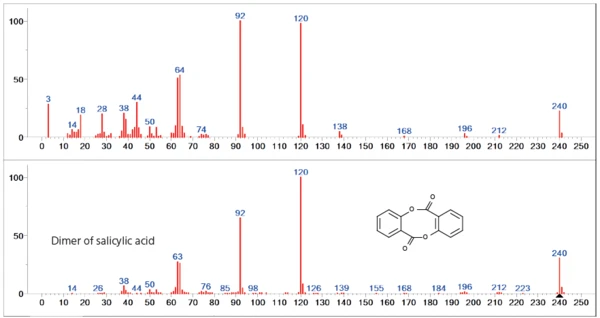
The second mass-loss step is mainly dominated by the release of the dimer of salicylic acid. Especially in the lower mass range (< 60u), however, differences to the database spectrum can be seen, indicating a release of additional gas species (figure 4).
Due to the outgassing products detected, a temperature-dependent view of the outgassing behavior can be compiled. To this end, specific mass numbers of the individual outgassing products were selected and plotted in comparison with the mass-loss curve (figure 5). The corresponding presentation illustrates overlapping of the thermal degradation of acetylsalicylic acid and evaporation of salicylic acid formed as a decomposition product within the first mass-loss step. It also becomes obvious that the formation and evaporation of the oligomers of salicylic acid already starts in the same temperature range and is the dominant degradation process in the following temperature course.
Summary
The combination of thermogravimetry and mass spectrometry is a powerful tool for obtaining deep insight into thermal decomposition processes and the gases released. Coupling with a mass spectrometer allows for an overview of temperature-dependent outgassing products that is of similarly high quality to the method of combining thermogravimetry with infrared spectroscopy. Due to the more specific character of the mass spectra, however, coupling with a mass spectrometer allows for more precise conclusions to be drawn with regard to the gas species released.
In summary, the thermal decomposition of acetylsalicylic acid in a helium atmosphere occurs in a two-step process composed of the separation of the acetyl functionality and the associated release of acetic acid along with the evaporation of salicylic acid in an oligomer form (e.g., dimer). A gas-analytical view of the respective mass-loss steps evidenced partial overlapping of the two processes due to the simultaneous release of acetic and salicylic acid within the first mass-loss step.
Detailed analysis of the MS spectra obtained suggests that not all outgassing products can be accessed by means of the direct coupling of TGA to a mass spectrometer. Thus, especially in the second loss step, it was only possible to clearly assign parts of the mass numbers observed. However, the combination of gas chromatography and mass spectrometry (GC-MS), as already shown in part 3 of this application note series, features an even more specialized coupling methodology, which was particularly developed for this kind of task [4].