Introduction
Clavulanic acid is used in combination with penicillin-group antibiotics because it overcomes antibiotic resistance in bacteria that secrete ß-lactamase, which otherwise inactivates most penicillins. It is commonly used in the form of potassium salt, potassium clavulanate [1].
Knowledge about the degradation of potassium clavulanate is crucial for improving its stability and thus its shelf life, which refers to the time interval that a drug product may be stored without becoming unfit for use, consumption or sale [3].
In the following, an investigation of the thermal behavior of potassium clavulanate by means of DSC and TGA is described.
Measurement Conditions
For the measurement with the NETZSCH DSC 204 F1 Phoenix®, the sample (2.67 mg) was heated in a sealed aluminum pan with pierced lid at a heating rate of 10 K/min under a nitrogen atmosphere (40 ml/min) in the temperature range between -80°C and 250°C. The TGA measurement was performed under the same conditions using the NETZSCH TG 209 F1 Libra® between room temperature and 600°C. The sample mass amounted to 5.34 mg.
Test Results
Figure 2 depicts the DSC measurement in the temperature range between room temperature and 220°C. The first DSC peak at 77°C is associated with a mass loss of 1.8% observed in the TGA measurement (figure 3). The shape of this broad effect, its temperature range, and the fact that it is an endothermal reaction indicate the release of surface water.
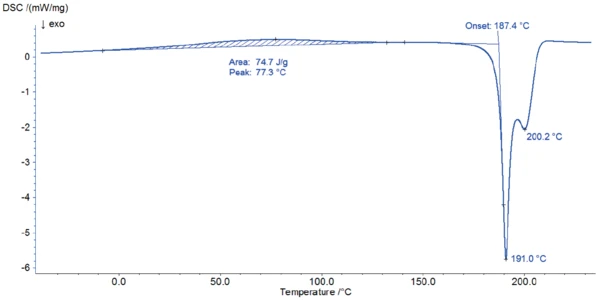
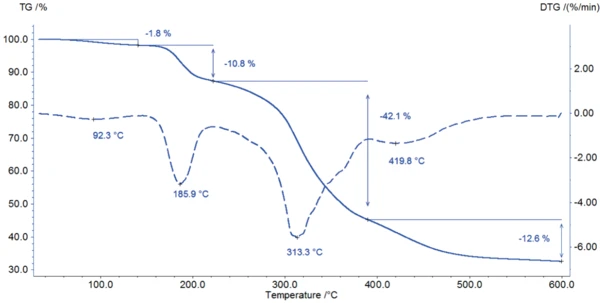
The start of the degradation of potassium clavulanate can be detected with both methods: In the DSC curve, a sharp EsotermicoUna transizione o reazione è esotermica se il materiale genera calore exothermal effect starts at 187°C (onset temperature); at this temperature, the TGA measurement records a mass loss of 11%.
The degradation continues with a mass loss of 42% between 200°C and 400°C (figure 3). Between 400°C and 600°C, another mass loss of 13% is reached at a maximum Reazione di DecomposizioneLa decomposizione è la reazione indotta termicamente con cui una sostanza chimica genera prodotti solidi e/o gassosi. decomposition rate at 420°C.
Conclusion
The heating of potassium clavulanate to 600°C begins with the evaporation of adsorbed water. Thereafter, the substance degrades in three steps at maximum decomposition rates at 187°C, 313°C and 420°C. DSC and TGA are complementary methods. A mass loss in the TGA curve associated with an endothermal effect in the DSC curve indicates a release of volatiles. On the other hand, the combination of a mass loss with a sharp, exothermic peak in the DSC curve is rather due to degradation. This information can be confirmed by measurements using TGA coupled to an evolved gas analyzer such as the FT-IR system (see TGA-FT-IR analysis on potassium clavulanate in NETZSCH Application Note 118).