Introduction
Early high cultures already used willow bark as a remedy for fever and pain [1]; the Roman scholar, Plinius the Elder, regarded willow bark as a medicine and the Teutons and Celts produced extracts by cooking willow bark, the ingredients of which were chemically related to synthetic acetylsalicylic acid [2]. Although various chemists were able to produce salicin and salicylic acid in the 19th century, it was not until 1897 that Felix Hoffmann succeeded in synthesizing acetylsalicylic acid without impurities at BAYER’s headquarters in Wuppertal-Elberfeld, Germany. Kurt Wittauer (figure 2) tested this drug on patients in the following years until BAYER (figure 1) finally filed for the corresponding patent in 1921. The painkiller began its triumphant success around the world and today, BAYER produces more than 50,000 tons of acetylsalicylic acid per year [4].


Drugs containing the active ingredient acetylsalicylic acid are available in various pharmaceutical forms and are employed not only because of their analgesic effect but also due to their anti-inflammatory, antipyretic and antiplatelet properties.
Pure acetylsalicylic acid is a pure white powder that is poorly soluble in water, has a Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point of 136°C and decomposes at higher temperatures. Various methods of thermal analysis, infrared spectroscopy, and combinations of the two were employed in this work to investigate gaseous Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition products.
Methods and Preparation
Acetylsalicylic acid (CAS: 50-78-2) was acquired from Sigma Aldrich with a purity of > 99%. For the investigation of the original substance, the BRUKER TENSOR II was used to measure the samples with attenuated total reflection (ATR). For determination of the melting behavior, the NETZSCH DSC 214 Polyma was used. For thermal characterization of the gases released, a thermobalance was coupled to an infrared spectrometer – the NETZSCH TG 209 F1 Libra® to the Bruker Equinox 55/S. The measurement conditions for the thermoanalytical and spectroscopic investigations are summarized in tables 1 to 3.
Table 1: Measurement conditions for the DSC investigation of acetylsalicylic acid
| Acetylsalicylic Acid | |
|---|---|
| Sample mass | 2.08 mg |
| Crucible material | Aluminum, pierced |
| Crucible mass | 52.75 mg |
| Temperature range | 25 ... 160°C |
| Heating rate | 7 K/min |
| Atmosphere | Nitrogen (50 ml) |
Table 2: Measurement conditions for the thermogravimetric investigation of an Aspirin® tablet by means of TGA-FT-IR
| Aspirin® | |
|---|---|
| Sample mass | 9.141 mg |
| Crucible material | Alumina, open |
| Crucible mass | 162.75 mg |
| Temperature range | 25 ... 600°C |
| Heating rate | 10 K/min |
| Atmosphere | Nitrogen (40 ml) |
| Scans | 32 |
| Resolution | 4 cm-1 |
| Spectral range | 650 - 4500 cm-1 |
Table 3: Measurement conditions for the spectroscopic investigation of (ATR) of acetylsalicylic acid
| Acetylsalicylic Acid | |
|---|---|
| Detector | DTGS |
| Scans | 32 |
| Resolution | 4 cm-1 |
| Spectral range | 650 - 4500 cm-1 |
Results and Discussion
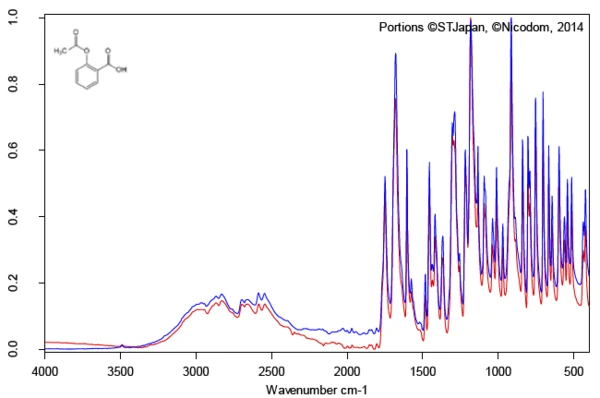
Investigation of the active ingredient acetylsalicylic acid with the help of FT-IR spectroscopy yields an infrared spectrum at room temperature which is in good agreement with the library spectrum (Bruker ATR-LIBPolymers-1-472-2) (figure 3). The melting range of acetylsalicylic acid is indicated by the manufacturer to be at 134°C to 136°C. The investigation by means of Differential Scanning Calorimetry (DSC) provides a melting enthalpy of 178 J/g and a temperature for the extrapolated onset of 138.5°C. As can also be clearly seen from figure 4, the heat-flow signal indicates the beginning of the melting process of the sample already at significantly lower temperatures than determined by the standardcompliant evaluation for the extrapolated onset. In literature, two polymorphic forms of acetylsalicylic acid are described: Form I with a Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature of 144.9°C and Form II with a Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature of 135.5°C [5, 6].
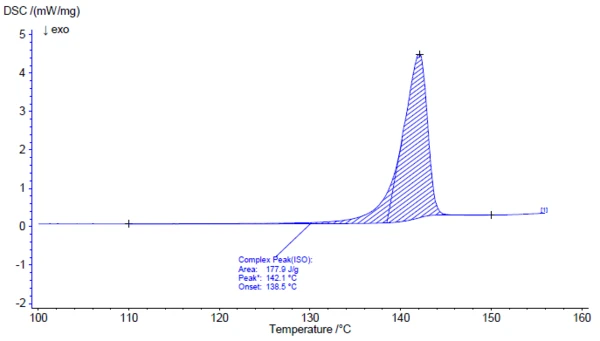
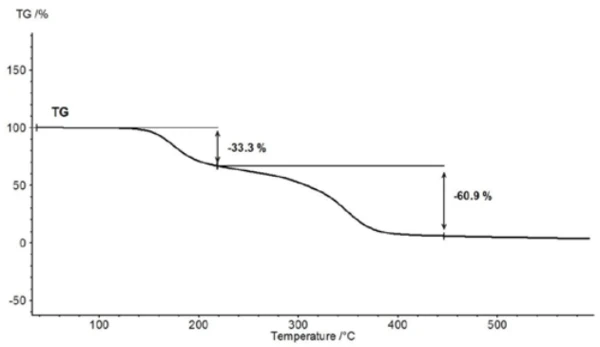
Above about 150°C, the thermal degradation of acetylsalicylic acid begins. Therefore, thermogravimetry (TGA) is better suited for further characterization above the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point (figure 5).
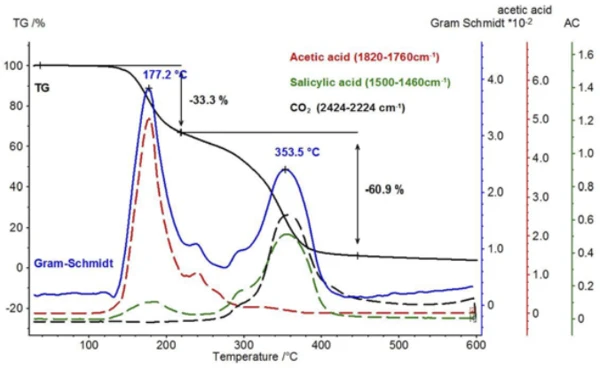
For characterization of the thermal degradation, a piece of an aspirin tablet was investigated with the help of the TGA-FT-IR coupling. Although the thermogravimetric results between 150°C and 450°C show a two-step thermal degradation reaction and the amounts of gases released can be quantified, it is not possible to determine which gases are responsible for the detected mass loss without spectroscopic analysis. If one carries out a measurement where the thermobalance is coupled to an infrared spectrometer, the gas phase can be investigated continuously during the entire measurement. All infrared spectra are presented in a three-dimensional arrangement, temperature-scaled, in figure 6. The results of the thermogravimetric measurement can also be seen in the left rear area.
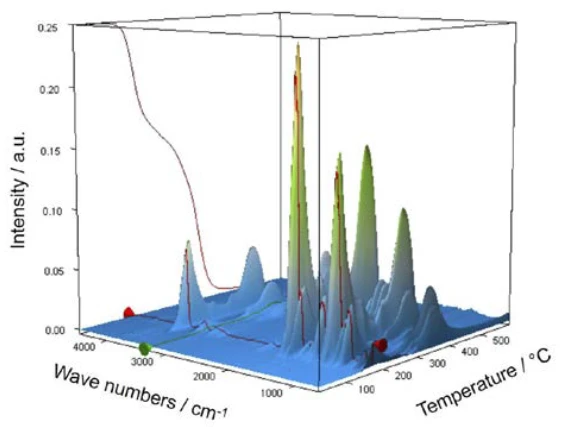
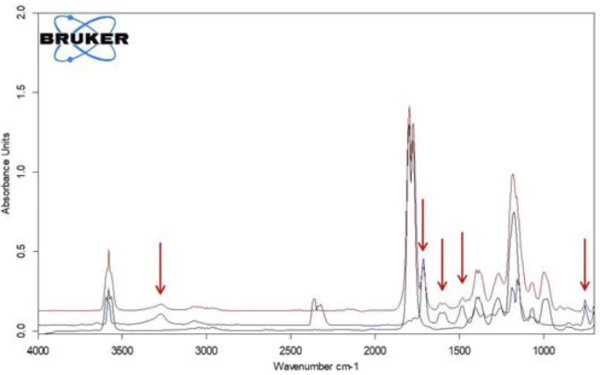
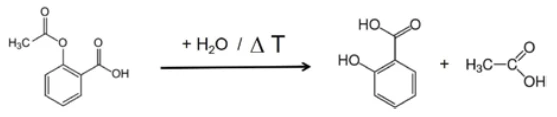
If individual spectra are extracted from this presentation at the temperatures with the highest absorption intensities, the gases released can be identified with the help of comparison spectra from gas phase libraries. The individual spectrum for the first mass-loss step at 180°C, which is characteristic, is in very good agreement with the spectrum for acetic acid from the EPANIST gas phase library (figure 7). The red arrows indicate absorption bands that do not match acetic acid, but correspond very well with the absorption bands for salicylic acid (EPA-NIST). This leads to the assumption that the acetylsalicylic acid, as in reaction equation 1, thermally degrades into salicylic acid and acetic acid (Equation 1). At 180°C, the acetic acid formed is already gaseous while the salicylic acid, with a Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point of 159°C, begins to evaporate. This is certainly also the reason why the first mass-loss step passes directly into the following step. The combination of Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition and evaporation confirms the degradation mechanism proposed by Rebeiro et al. [7]. In conjunction with the tablet form of the active ingredient acetylsalicylic acid, the influence of humidity on the reaction products of the thermal degradation is emphasized along with additives such as starch and magnesium stearate monohydrate. Gupchup et al., however, point out that the dry active ingredient acetylsalicylic acid can itself ensure the presence of water through dimerization in the sense of condensation [8].
When comparing the two spectra for acetic acid and salicylic acid, it is noticeable that the absorption bands in the range between 1760 cm-1 and 1820 cm-1 can only be attributed to acetic acid, while the absorption bands between 1460 cm-1 and 1500 cm-1 represent salicylic acid. If the intensity course of the absorption ranges is calculated as a function of temperature, “traces” are obtained for each substance; these are proportional to the corresponding amounts released as a function of temperature.
(Equation 1)
A comparison of these temperature-dependent traces for acetic acid and salicylic acid is shown in figure 8 with the Gram-Schmidt trace (sum of the intensities that are not dependent upon wavelength) and the TGA signal. As with the TGA signal, the Gram-Schmidt trace reveals that the first mass-loss step passes directly and without plateau into the second mass-loss step. The reason for this can be found in the traces of the two products, which show that the release of acetic acid can be detected up to about 300°C and, in addition, the evaporation of salicylic acid starts already at lower temperatures.
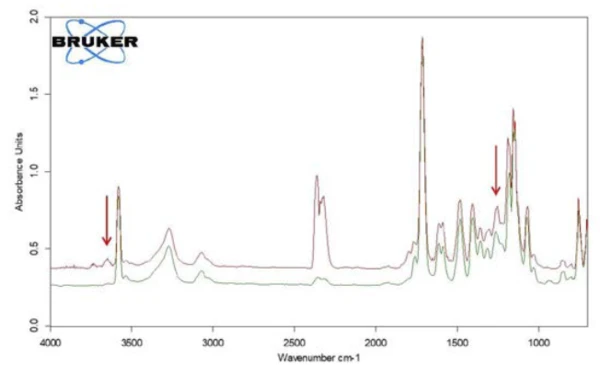
Along with salicylic acid, also the formation of carbon dioxide can be detected by means of the temperaturedependent course of the absorption intensities. This is confirmed by the extracted individual spectrum at 360°C (figure 9).
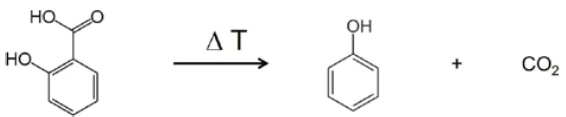
In the range between wave numbers 2424 and 2224, the absorption bands of CO2 are clearly visible. In addition, there are indications that phenol has formed. The positions of the most intense absorption bands for phenol are marked with red arrows. It can therefore be assumed that – along with the evaporation of salicylic acid – a Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition process also takes place; this suggests the formation of phenol and CO2 as shown in equation 2.
(Equation 2)
Summary
Acetylsalicylic acid was investigated using FT-IR spectroscopy at room temperature (ATR), and the FT-IR spectra obtained were used for identification by means of comparison with a spectra library. The DSC was used for investigation of the melting behavior. Additionally, the thermal behavior of Aspirin® was characterized by means of TGA-FT-IR. The spectra for the gases released during thermal treatment were compared with a gas phase library for identification of the products. It was thus possible to confirm degradation mechanisms known from literature and it was additionally shown that the common additives used in the tableting of Aspirin® appear to have no detectable influence on the formation of gaseous Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition products.