Metals & Alloys
Steel Material - Phase Transitions
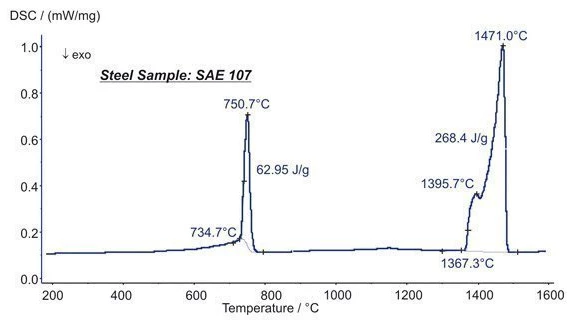
Presented here are the transformation energetics of a steel (SAE 107).
At 751°C, two Phase TransitionsThe term phase transition (or phase change) is most commonly used to describe transitions between the solid, liquid and gaseous states.phase transitions overlap each other. The increase in the heat flow rate up to 735°C is due to the Curie transition (change in the magnetic properties). A change in the crystal structure (bcc to fcc structure) is causing the major peak. The structural change is connected with an enthalpy change of 63 J/g. Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).Melting was seen at 1367°C and occurred in two steps (peaks at 1395°C and 1471°C). The heat of fusion was 268 J/g. (measurement with DSC 404 F1 Pegasus®®)