tips & Tricks
Not Even Platinum is Everlasting!
Thermocouples have become established as standard temperature measuring devices in thermal analysis: they feature simple set-up and operation, and they are multifunctional, robust and compact.
The most frequently used thermocouple material for operation above 800°C is platinum-platinum /rhodium (10% ) – with regard to its chemical composition, also designated Pt-Pt 10% Rh,- or also referred to as type S. The main advantages of this thermocouple, developed by Le Chetalier more than 100 years ago, are high reproducibility, good corrosion and oxidative stability.

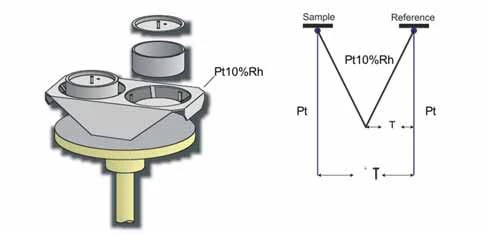
Set-up:
The negative side of the thermocouple consists of platinum ; the positive – in accordance with ASTM E1159 – of platinum/rhodium with a weight proportion of approx. 10.00+/- 0.05% rhodium.
Resistance:
Compact platinum-platinum/rhodium features a virtually unlimited resistance at room temperature. This, however, changes under regular operation at high temperatures. Interdiffusion, selective evaporation, recrystallization and environmental influences are the main reasons for changes in thermal tension or failure of the thermocouple.
a) Selective evaporation and interdiffusion
At temperatures above 1000°C, evaporation of rhodium and also diffusion of rhodium from the positive Pt 10% Rh side to the negative Pt side occur. Both effects result in impurities and increased wear and tear of the platinum wire. In order to minimize the risk of alloy formation over the gas phase, the majority of the thermocouple wire for DSC/DTA sample carriers is protected by a capillary of high-purity Al2O3.

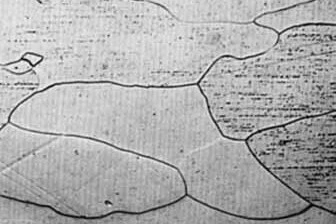
b) Recrystallization
In the temperature range above 1100°C, platinum recrystallizes into a coarse-grained structure. The described grain growth not only occurs within the metal or metal alloy, but also leads to “coalescence”of different platinum parts which are in contact with each other, such as DSC/TG sensor type S and Pt/Rh DSC crucibles. Only conditioning of new sample carriers and crucibles by means of a special thermal treatment lowers the “sticking tendency”.
Using unconditioned sample carriers and crucibles in the temperature range above 1000°C immediately leads to welding of the crucible onto the sensor and thus, destruction of the sample carrier.
Please pay attention to the instruction leaflet of your sample carrier in this regard. We would ask you to heat new Pt/Rh crucibles prior to using them in a separate furnace to the required end temperature of the measurement, lift the crucibles from the sensor after each measurement as a precaution, and go up to temperatures above 1100°C only stepwise in the beginning.
In our experience, using dispersion-hardened (so-called FKS) materials for the sensor surfaces and crucibles does not result in a long-term significant improvement.
One way of avoiding the described phenomenon is to underlay thin disks (between sensor surface and crucible). The risk of sticking is minimized and the sensitivity of the sample carrier is only slightly decreased.
c) Environmental effects
In practice, the biggest influence on the service life of thermocouples is due to interactions with the environment. Diffused impurities, released from the samples, change the thermal tension or may even cause initial cracking of the thermocouple wire. In the table, you will find details on the chemical compatibility of platinum with other sample materials and gas atmospheres.

This list demonstrates how important regular inspections and calibration measurements are. This is the only way to ensure that the employed thermocouple material Pt-Pt10% Rh does not exceed the defined tolerance limit over a longer period of time.
Critical for platinum:
- Halogens (Cl2, F2, Br2), aqua regia
- Li2CO3, prior to emission of CO2 (Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition)
- PbO, FeCl2
- Be alloys (evaporation)
- HCl with oxidants (e.g., chromic acid, manganates, iron (III) salts and molten salts); reducing atmospheres
- Metals and metal vapors (e.g., B, Pb, Zn, Sn, Ag, Au, Li, Na, K, Sb, Bi, Ni, Fe, etc.;m Se > 320°C (evaporation)
- Metals and metal oxides with reducing sub- stances such as C, organic compounds or H2
- Oxides in an inert gas atmosphere at higher temperatures (reduction)
- Sulfur (roughening of the surface, embrittlement)
- Alkali hydroxides, -carbonates, -sulfates, -cyanides and rhodanides at higher temperatures
- KHSO4 at higher temperatures
- Carbon black or free carbon >1000°C
- SiO2 under reducing conditions
- SiC and Si3N4 >1000°C (release of elementary Si)
- HBr, KCl solution at high temperatures
(No claim of completeness)
No resistance to:
- Mixtures of KNO3 and NaOH at 700°C under exclusion of air
- Mixtures of KOH and K2S at 700°C under exclusion of air
- LiCl at 600°C
- MgCl2, Ba(NO3)2 at 700°C
- HBr, HJ, H2O2 (30%) and HNO3 at 100°C
- KCl (the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition products which form during melting; Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point:768°C)
Limited resistance to:
- KHF2, LiF2, NaCl at 900°C
- Mixtures of NaOH and NaNO3 at 700°C under exclusion of air
No claim is made that this overview is exhaustive; it is only a guideline for the user. For the most part, the temperatures are literature values. The temperatures under test conditions might be shifted to lower values. It is always advisable to run preliminary tests in separate furnaces. NETZSCH-Gerätebau excludes liability for any damages resulting from improper use of the instruments, crucibles, sample carriers, etc.