Introduction
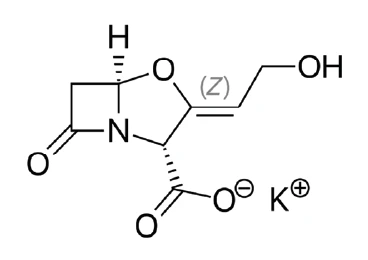
Potassium clavulanate (figure 1) is a salt of clavulanic acid which is a major ß-lactam antibiotic produced by the organism Streptomyces clavuligerus [1]. On its own, it is actually only capable of weak antibacterial activity against most organisms, but in combination with the antibiotic amoxicillin, it is effective against ß-lactamaseproducing staphylococcus bacteria which are resistant to amoxicillin alone [2, 3]. That is why it is an established substance in the pharmaceutical industry.
Amoxicillin and potassium clavulanate exhibit similar Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition pathways. However, the stability of the amoxicillin-clavulanate combination mainly depends on the clavulanate, which is the more degradable of the two [4, 5].
The Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of potassium clavulanate has been investigated in many papers [3, 4, 7, 12]. Generally, the substance was studied in solutions with different pH levels and in the presence of amoxicillin. It was observed that the stability of the admixture amoxicillin/clavulanic acid is affected by an increase in temperature from 25°C to 40°C [3]. On the other hand, the shelf-life of the admixture increases significantly if the pH of the solution is acidified [4]. It was also observed that in solutions, the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of clavulanic acid is catalyzed by the products of hydrolysis [12]. As is shown by using the HPLC method on samples stored at different temperatures and under different atmospheric conditions, the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of potassium clavulanate in the solid state follows another mechanism: The Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition products formed in the solid phase do not have any catalytic effect [8].
The Thermal StabilityA material is thermally stable if it does not decompose under the influence of temperature. One way to determine the thermal stability of a substance is to use a TGA (thermogravimetric analyzer). thermal stability can also be explored with thermogravimetry, which determines, amongst other things, the temperature at which a material starts to decompose or react [9]. The thermal decomposition of solid potassium clavulanate was characterized by means of a thermobalance coupled to an FT-IR spectrometer in [13]. In the following, thermogravimetric measurements are used to carry out kinetic studies of the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition reaction.
This allows for the prediction of the potassium clavulanate degradation for specific temperature and time conditions. Knowledge of the Thermal StabilityA material is thermally stable if it does not decompose under the influence of temperature. One way to determine the thermal stability of a substance is to use a TGA (thermogravimetric analyzer). thermal stability and understanding the decomposition process of potassium clavulanate in the solid state allows for optimizing its storage conditions.
Experimental
The TGA measurements were performed with a NETZSCH TG 209 F1 Libra® thermobalance with automatic sample changer. From the TGA-FT-IR measurement described in [13], we learned that the sample releases surface water as soon as the measurement starts. For this reason, the next measurements were carried out using closed aluminum crucibles. Just prior to the measurement, the crucible lid was automatically pierced by the piercing device of the ASC. This prevents the sample from releasing its surface water already before the actual measurement starts, which would falsify the value of the initial mass.
The sample masses were between 4.33 and 5.04 mg. The samples were heated between room temperature and 600°C at four heating rates varying from 1 K/min to 10 K/min. The measurements were carried out in a dynamic nitrogen atmosphere (40 ml/min).
The TGA curves obtained are the basis for the kinetic evaluation of the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition reaction.
For this, the Kinetics Neo software (by NETZSCH-Gerätebau GmbH) was used. It allows for modelling the kinetics of single to multi-step reactions.
This software can assign each individual step to different reaction types with kinetic parameters of their own, such as activation energy, order of reaction, and pre-exponential factor. Based on the results, Kinetics Neo is able to simulate the reaction(s) for user-specified temperature programs.
Results and Discussion
TGA Measurements
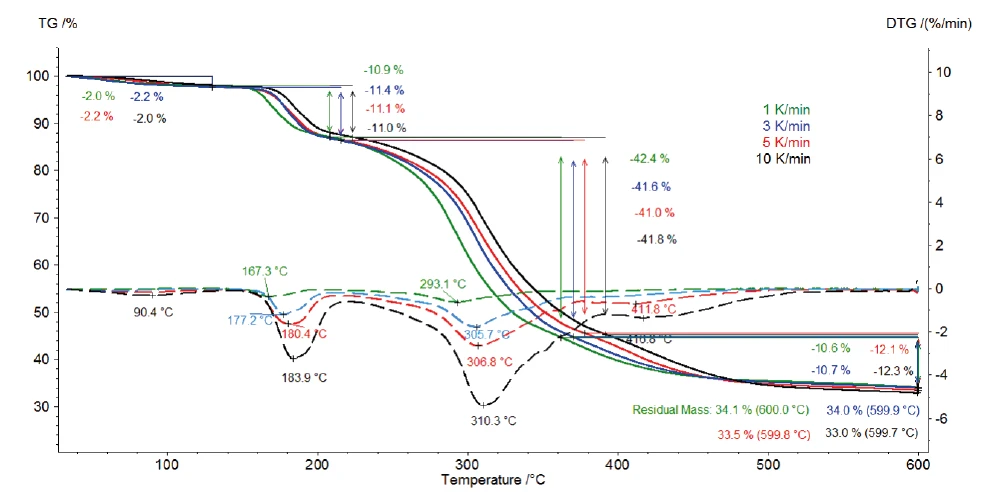
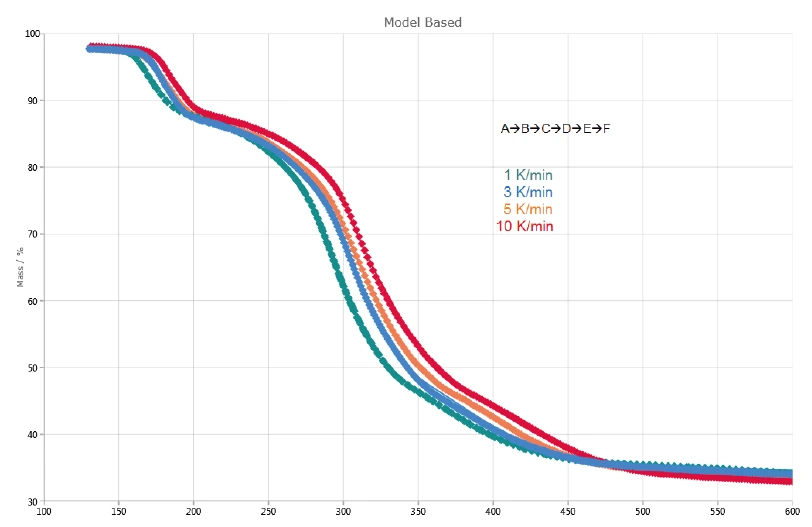
Figure 2 depicts the TGA and DTG (first derivative) curves of the measurements on potassium clavulanate at heating rates of 1, 3, 5 and 10 K/min. The first mass-loss step, detected between room temperature and 120°C, results from the evaporation of surface water [13]. Further, the three mass-loss steps identified between 120°C and 600°C are due to the decomposition of potassium clavulanate. They are shifted to higher temperatures with increasing heating rates (kinetic influence). For example, at a heating rate of 1 K/min, the first decomposition step occurs at 167°C (DTG peak), while at a heating rate of 10 K/min, it occurs at 184°C (DTG peak). The last decomposition step becomes more pronounced with increasing heating rate: At a heating rate of 5 K/min, a DTG peak is observed at 412°C (red dashed curve), while at 10 K/min, it occurs at 417°C (black dashed curve).
Kinetic Analysis of the Thermal Decomposition
The dependence of the decomposition on the heating rate allows for evaluation of the process with the help of NETZSCH Kinetics Neo software. Figure 3 shows the TGA measurement curves between 130°C and 600°C used for the kinetic evaluation. The release of surface water at temperatures lower than 130°C is not taken into consideration.
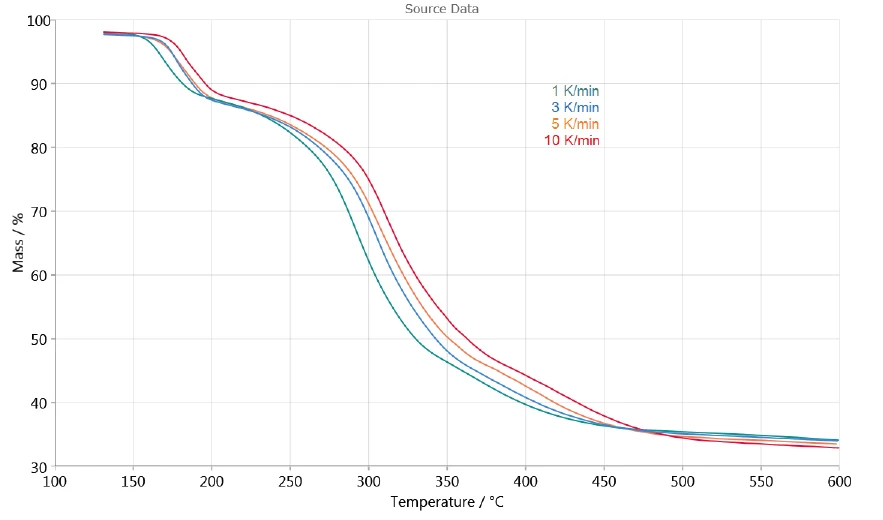
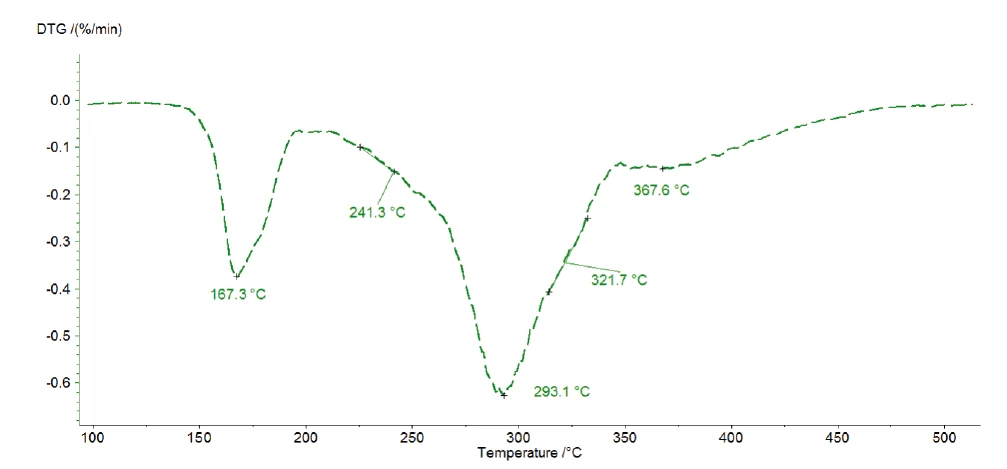
The three consecutive mass losses detected indicate at least three decomposition steps. The DTG curve of the measurement at 1 K/min presented in figure 4 shows three peaks at 167°C, 293°C and 368°C, but also two shoulders with onset temperatures at 241°C and 322°C. That is the reason why Kinetics Neo proposes a kinetic model with five consecutive steps of nth order.
The reaction rate of each step j is described by the function: Reaction Ratej = Aj · f(ej,pj) · exp[-Ej/RT]
Aj: pre-exponential factor
Ej: activation energy [J.mol-1]
T: temperature [K]
R: gas constant (8.314 J.K-1.mol-1)
f(ej,pj): function dependent on the concentration of the
initial reactant ej and the concentration of product pj
Figure 5 compares the measured TGA curves (dotted lines) with the calculated curves (solid lines) of the chosen 5-step model. A high correlation coefficient, of >0.999, is achieved between the measured and calculated data.
Table 1 summarizes the results of the kinetic evaluation for each step. The theoretical mass loss is calculated by multiplying the contribution of the reaction step to the decomposition with the total mass loss occurring during decomposition.
The first decomposition step, A→B, is associated with a calculated mass loss of 11.9% and corresponds to the experimental values of 11%. The mass loss for the last step, E→F, amounts to 13.9%. This is slightly higher than the experimental value of 11 - 12%. It means that the last mass-loss step starts earlier (< 360°C). The total mass loss of steps B→C, C→D and D→E is 36.9% and corresponds to the complex decomposition process around 300°C (DTG peak) in figure 2.
Tab 1: Kinetic parameters of the thermal degradation of potassium clavulanate
| Reaction step | A → B | B → C | C → D | D → E | E → F |
|---|---|---|---|---|---|
| Activation energy [kJ/mol] | 265.1 | 240.8 | 260.5 | 179.8 | 166.5 |
| Pre-exponential factor | 28.6 | 21.6 | 21.7 | 13.3 | 10.5 |
| Reaction order | 3.6 | 2.1 | 1.8 | 1.6 | 3.4 |
| Contribution | 0.190 | 0.099 | 0.244 | 0.246 | 0.222 |
| Theoretical mass loss | 11.9% | 6.2% | 15.3% | 15.4% | 13.9% |
The good correlation of the measurements with nth order reactions confirms the conclusions drawn in [8] that contrary to its decomposition behavior in solutions, the decomposition of potassium clavulanate in a solid state is not auto-catalyzed.
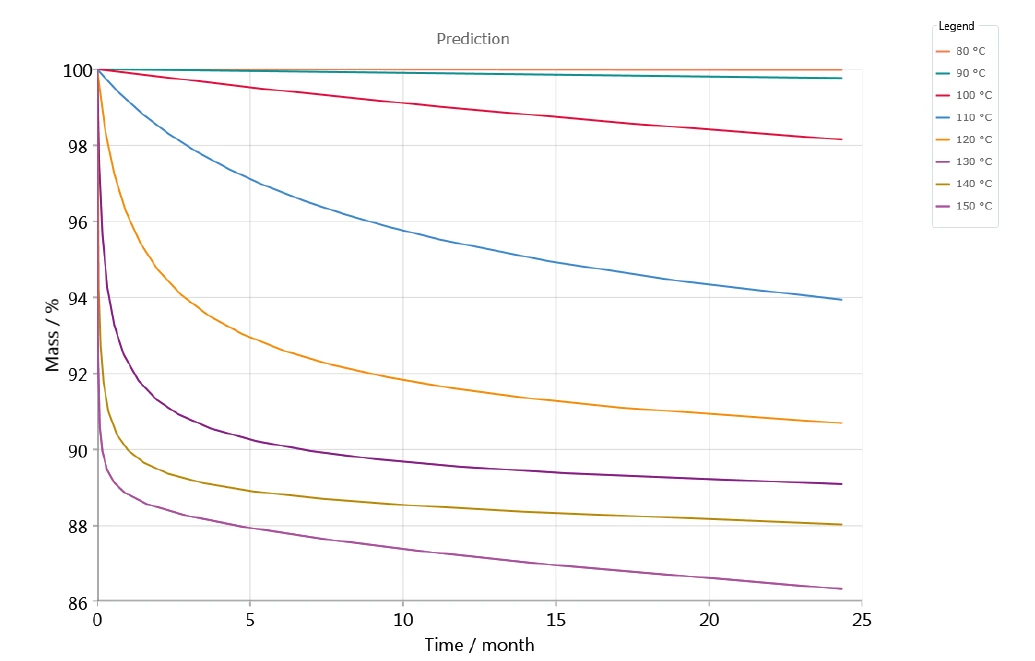
The kinetic evaluation was carried out with a high correlation coefficient and thus a high level of agreement between the measured and simulated TGA curves, so that predictions about the long-term behavior under different storage temperatures are possible. As an example, figure 6 shows the mass change versus time based on the 5-step model with consecutive steps; it represents the prediction of the decomposition of potassium clavulanate for various temperatures between 80°C and 150°C in a nitrogen atmosphere. With increasing temperature, the decomposition increases. This effect can already be observed at a storage temperature of 90°C (green curve at the upper end of the graph – figure 6).
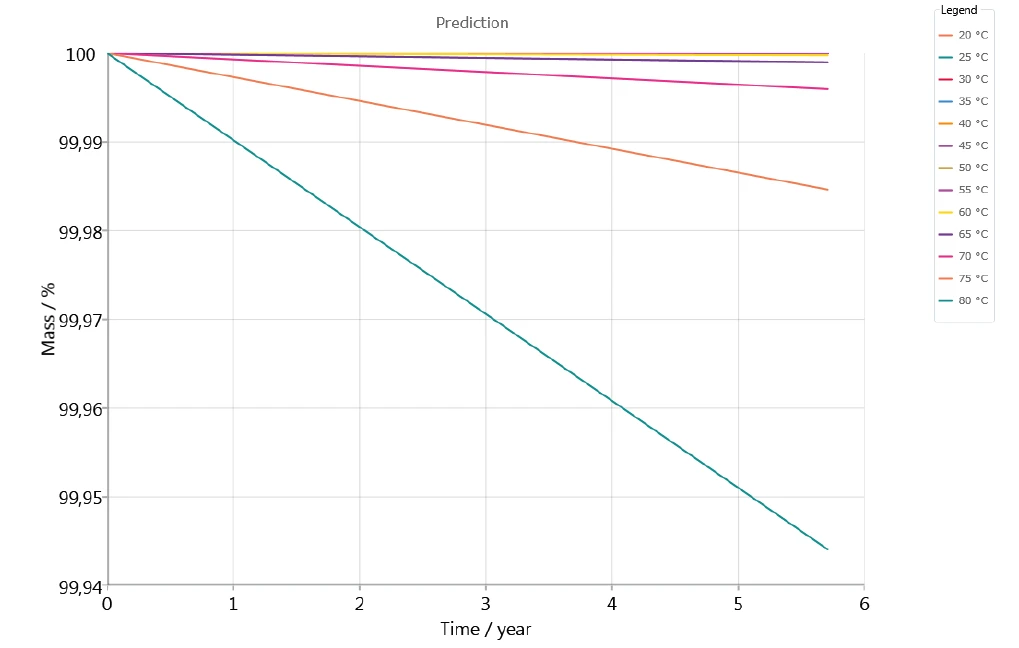
Figure 7 depicts the stability in an inert atmosphere of the drug over the course of 5 years, for temperatures between 20°C and 80°C. It seems that no significant mass loss occurs in the prediction for temperatures up to 60°C.
A reminder should be inserted here that the decomposition kinetic was carried out on a dry sample. However, water has great influence on the decomposition of potassium clavulanate: Storage in a humid atmosphere shifts its decomposition to lower temperatures [10]. J. Cieleka-Piontek shows that potassium clavulanate samples decompose faster if exposed to increased air humidity than when they are exposed to dry air and suggests that the attack of a water molecule on the carbonyl group of the ß-lactam ring induces thermolysis [8].
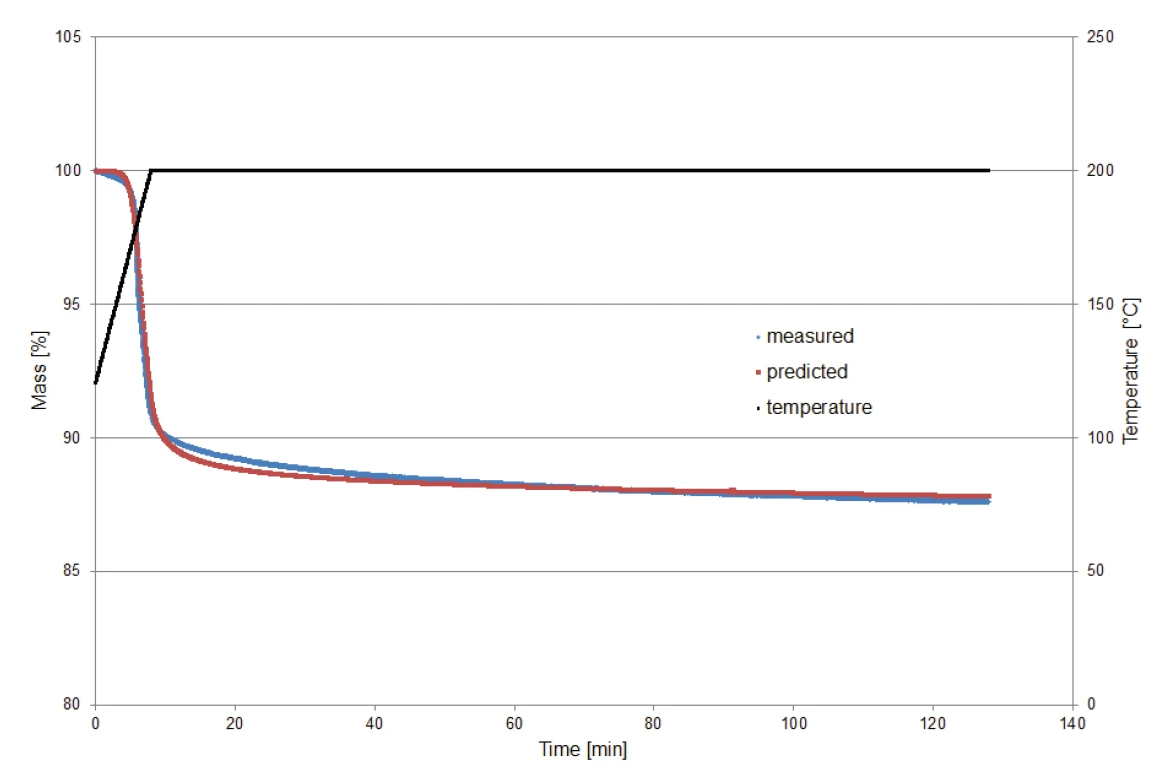
In order to validate the kinetic model calculated by Kinetics Neo for prediction of the decomposition behavior under IsothermalTests at controlled and constant temperature are called isothermal.isothermal conditions, a potassium clavulanate sample of 9.23 mg was heated to 200°C and then kept IsothermalTests at controlled and constant temperature are called isothermal.isothermal for two hours. Monitoring of the measurement started at 120°C in order to exclude the mass-loss effect of the release of surface water.
Figure 8 compares the mass losses determined via measurement to those determined via prediction (Kinetics Neo). The comparison shows the good agreement between the two curves and thus the reliability of the calculation.
Conclusion
The kinetics of the thermal decomposition of potassium clavulanate in a solid state under nitrogen were investigated by means of thermogravimetry and Kinetics Neo. A high level of correlation between measured and simulated data can be obtained by using a consecutive five-step kinetic model where each step is of nth order. This allows for predictions of the storage behavior under different temperatures, temperature profiles and periods.
The results are validated by comparing the TGA measurement under a specified temperature profile including IsothermalTests at controlled and constant temperature are called isothermal.isothermal segment to the predictions calculated by Kinetics Neo.