Introduction
Lactose is a sugar found in the milk of mammalians. It can be obtained in an amorphous or a crystalline form. α-lactose crystallizes as a monohydrate while ß-lactose does not contain crystal water; therefore, it is often described as anhydrous lactose. A particular form of lactose is obtained by spray drying of a solution of finely milled α-lactose monohydrate. During this process, amorphous lactose is formed in addition to crystalline lactose. The obtained product is a matrix of lactose glass in which lactose monohydrate crystals with narrow size distribution are embedded. The presence of the amorphous structure facilitates the compression processes and leads to better tableting properties [1, 2, 3].
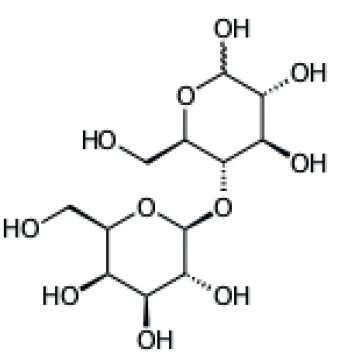
Lactose, Humidity and Caking
The affinity of lactose products to humid air depends on their modification. Pure α-lactose monohydrate products are very stable to humid air. In contrast, amorphous lactose is very hygroscopic: At a certain humidity, amorphous lactose is transformed into the crystalline α-lactose monohydrate form and exhibits changes in its compression properties [2].
Caking (appearance of lumps of various sizes in lactose powder) is a common problem that can occur during production, storage or transport of powders. If a powder is caked, this results in longer processing times and reduced product quality. Caking results from the formation of solid bridges between particles due to humidity, temperature fluctuations, pressure and migration of small particles [4, 5]. The ability to cake also depends on the particle size distribution. For example, small lactose crystals with a particle size of less than 300 μm can cake easily as soon as the water content is higher than 3% [4].
In the following, the influence of moisture on the storage behavior of lactose FlowLac® 90 from MEGGLE is studied by means of TGA. FlowLac® 90 is a spray-dried α-lactose monohydrate containing 8% to 12% of amorphous lactose.
Measurement Conditions
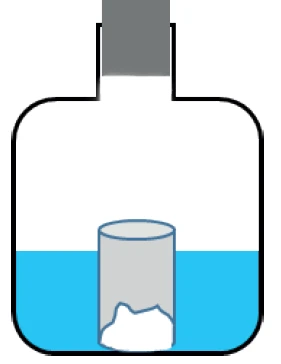
For the moisture treatment, the sample was stored in an open container placed in a closed vessel filled with water (no direct sample contact with water) for two weeks (figure 2).
The measurements were carried out with the TG 209 F1 Libra® under a dynamic nitrogen atmosphere (40 ml/min). Two lactose specimens were prepared in closed aluminum crucibles: one as received (6.43 mg) and the other one after a two-week storage period in a humid atmosphere (7.62 mg). The lid of each sample pan was automatically pierced from the instrument just before the measurement. The samples were heated from room temperature to 600°C at 10 K/min.
Test Results
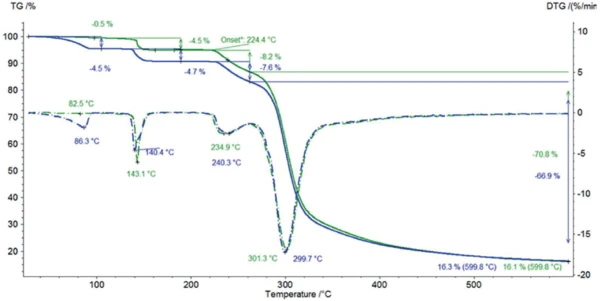
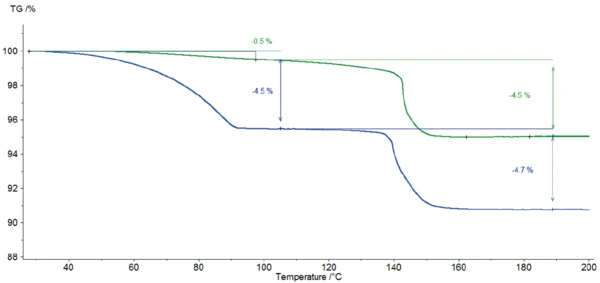
Figure 3 depicts the mass changes in both samples during heating to 600°C. Figure 4 displays a zoom of the temperature range from room temperature to 200°C. The two TGA curves differ significantly in the first massloss step resulting from the release of surface water: The two-week humidity storage yields an increase in adsorbed water from 0.5% to 4.5% (blue curves). No significant difference is detected in the second massloss step of 4.5% and 4.7%, respectively. This step is due to the release of crystal water present in the α-lactose monohydrate. It is followed by the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition at 224°C (extrapolated onset of the TGA curve) which takes place in two steps, independently of the moisture treatment. More information about the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition process is given in [7].
Conclusion
Thermogravimetry allows for the determination of surface water and CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization water in a single analysis. Classical methods for water determination, such as Karl Fischer, toluene distillation and conventional oven methods, require more analysis time in some cases and deliver fewer results than just a single TGA measurement [8]. Two-week storage in a humid atmosphere at room temperature results in a sharp increase in surface water in spray-dried α-lactose monohydrate. Here, the TGA method serves as a quality control tool by monitoring the amount of surface water in the product so that no powder caking occurs during the storage, transport and processing of lactose.