Introduction
The properties of worm-like micelles (WLMs) represent a key research area in both academia and industry. This is primarily due to the fact that they have widespread applications across a range of industries ranging from personal care to oil recovery. They offer a simple, cost effective way to generate remarkable viscosity and viscoelasticity. They can be made into ‘smart’ or stimuli responsive structures that can undergo transitions into another phase with strikingly different rheology. Such a response is of high interest for biomedical and drug delivery applications and also for separations using microfluidic devices.
Worm-like micelles can be formed from a wide range of different surfactant systems (anionic, cationic and zwitterionic) and also from various block copolymers. The key interesting factor is that although they can be formed from such a wide variety of chemical species, their rheological response is strikingly similar and they have a distinct rheological signature. The theoretical developments, which are now well established and widely accepted, allow not only the detection of the structure (as revealed through the distinct rheological signature), but also allows the extraction of important structural parameters.
This allows researchers to gain insight into how various formulation conditions such as electrolyte level, pH or surfactant composition impact on the microstructure of the formed worm-like micelle. Worm-like micelles are in a vast majority of cases formed from surfactants, which are amphiphilic molecules. Depending upon the surfactant packing parameter, surfactants can assemble into a wide variety of microstructures (see Table 1).
Table 1: Impact of packing parameter on the formed surface microstructure
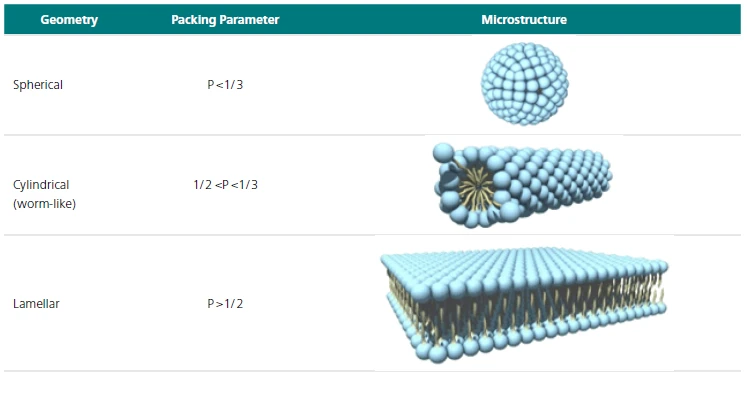
When the packing parameter is between 1/2 and 1/3, then the surfactant molecules can arrange into a rod like micellar arrangement. Based on their thermodynamics, these rod-like micelles can continue to grow with increasing concentration or on addition of an electrolyte or co-surfactant into worm like micelles and then onto nematic liquid crystals (Figure 1).
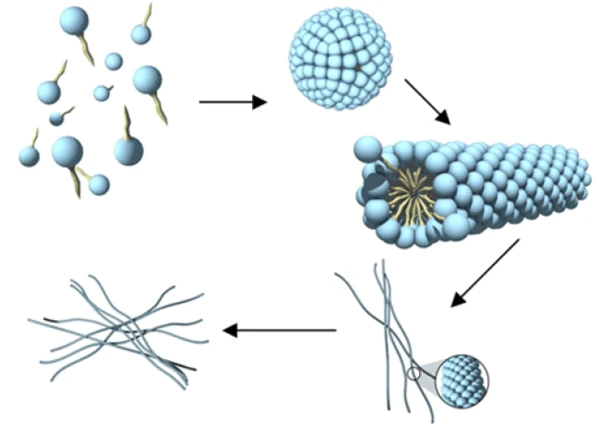
Each of the different phases illustrated in Figure 1 exhibits distinct rheological characteristics. The most pronounced and clear rheological signature is that of semi-dilute and concentrated worm-like micelle. Transitions from the dilute to the semi-dilute and from the concentrated to the nematic phases can also be followed through rheology.
Since they are the primary rheology building structures in a wide range of different applications, understanding their rheological signature and the changes in their structure and corresponding rheology on addition/changes in formulation is a key insight desired by both academic and industrial scientists. Rheology can provide specific insights into micellar growth, entanglement, branching, and shear induced transitions.
Theory
Worm-like micelles are similar to polymers, they are long and flexible, and their spectacular viscosity and viscoelasticity is driven by entanglement of the worm-like micelles. Two key structural features, which control their rheological response is the contour length L (a measure of the end to end distance) and the persistence length lp (a measure of the flexibility of the micelle). The elasticity of the system is impacted by the hydrodynamic correlation length ξH of the worm-like micelle.
StressStress is defined as a level of force applied on a sample with a well-defined cross section. (Stress = force/area). Samples having a circular or rectangular cross section can be compressed or stretched. Elastic materials like rubber can be stretched up to 5 to 10 times their original length.Stress RelaxationWhen a constant strain is applied to a rubber compound, the force necessary to maintain that strain is not constant but decreases with time; this behavior is known as stress relaxation. The process responsible for stress relaxation can be physical or chemical, and under normal conditions, both will occur at the same time. relaxation in a worm-like micelle, similar to polymers, can take place by reptation, (StressStress is defined as a level of force applied on a sample with a well-defined cross section. (Stress = force/area). Samples having a circular or rectangular cross section can be compressed or stretched. Elastic materials like rubber can be stretched up to 5 to 10 times their original length.stress RelaxationWhen a constant strain is applied to a rubber compound, the force necessary to maintain that strain is not constant but decreases with time; this behavior is known as stress relaxation. The process responsible for stress relaxation can be physical or chemical, and under normal conditions, both will occur at the same time. relaxation through snake-like motion of a polymer through a tube formed by its neighbours, until it exits the tube, at which point the StressStress is defined as a level of force applied on a sample with a well-defined cross section. (Stress = force/area). Samples having a circular or rectangular cross section can be compressed or stretched. Elastic materials like rubber can be stretched up to 5 to 10 times their original length.stress is completely relaxed) and also by breaking and re-forming.
The reptation time is dependent on the volume fraction φ and is given by: τrep ~ L3φ3/4
The breaking/forming time is given by: τbreak ~ 1/L
When τbreak > τrep, the micelles behave very much like unbreakable polymers, with exponential polydispersity and the StressStress is defined as a level of force applied on a sample with a well-defined cross section. (Stress = force/area). Samples having a circular or rectangular cross section can be compressed or stretched. Elastic materials like rubber can be stretched up to 5 to 10 times their original length.stress RelaxationWhen a constant strain is applied to a rubber compound, the force necessary to maintain that strain is not constant but decreases with time; this behavior is known as stress relaxation. The process responsible for stress relaxation can be physical or chemical, and under normal conditions, both will occur at the same time. relaxation takes the form:
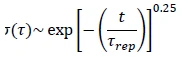
Equation 1
If τbreak < τrep , the RelaxationWhen a constant strain is applied to a rubber compound, the force necessary to maintain that strain is not constant but decreases with time; this behavior is known as stress relaxation. The process responsible for stress relaxation can be physical or chemical, and under normal conditions, both will occur at the same time. relaxation time is given by τ = (τbreak τrep)1/2. Under these conditions, the fluid behaves as a Maxwell fluid for which
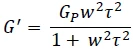
Equation 2
or
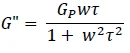
Equation 3
The zero shear viscosity η0 can be linked to the plateau modulus Gp by
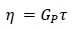
Equation 4
Hydrodynamic Correlation Length (ξH)
The hydrodynamic correlation length, ξH, can be extracted from the plateau modulus:
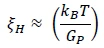
Equation 5
Where kB is Boltzmann Constant and T is temperature in Kelvins. The hydrodynamic correlation length is in nanometers.
Entanglement Length (le)
If the persistence length is estimated or extracted (from high frequency rheology through Microrheology or Small Angle Neutron Scattering), then one can calculate the entanglement length through
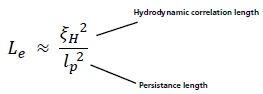
Equation 6
Experimental
- In this experiment a worm-like micelle structured bodywash was evaluated in order to determine its RelaxationWhen a constant strain is applied to a rubber compound, the force necessary to maintain that strain is not constant but decreases with time; this behavior is known as stress relaxation. The process responsible for stress relaxation can be physical or chemical, and under normal conditions, both will occur at the same time. relaxation time and hydrodynamic correlation length.
- Rotational rheometer measurements were made using a Kinexus rheometer with a Peltier plate cartridge and a cone and plate measuring system1, utilizing standard pre-configured sequences in the rSpace software.
- A standard loading sequence was used to ensure that the sample was subject to a consistent and controllable loading protocol.
- All rheology measurements were performed at 25°C.
- A frequency sweep test was performed between 0.2 and 40 rad/s using a StrainStrain describes a deformation of a material, which is loaded mechanically by an external force or stress. Rubber compounds show creep properties, if a static load is applied.strain value within the Linear Viscoelastic Region (LVER)In the LVER, applied stresses are insufficient to cause structural breakdown (yielding) of the structure and hence important micro-structural properties are being measured.LVER.
- A Cole-Cole plot (plot of G‘‘ vs G‘) was produced auto-matically from the frequency sweep to establish whether or not the characteristic semi-circular shape (Maxwell response) of the worm-like micelle was obtained.
- Values for Gp and τ were extracted from the frequency sweep data and ξH calculated from the former.
Results and Discussion
The frequency response of G’, G’’ for the bodywash product is shown in Figure 2(a) and the corresponding Cole-Cole plot is shown in Figure 2(b).
The data shown in Figure 2(a) are similar to those expected for a single RelaxationWhen a constant strain is applied to a rubber compound, the force necessary to maintain that strain is not constant but decreases with time; this behavior is known as stress relaxation. The process responsible for stress relaxation can be physical or chemical, and under normal conditions, both will occur at the same time. relaxation time Maxwell model with the onset of a plateau in G’ at high frequencies (Gp) and a cross-over in G’/G” at ωc = 1/τ. The semicircular shape of the Cole-Cole plot confirms Maxwell behavior. Most simple bodywash or transparent shampoo products generally conform to this behavior, the worm-like micelle structure resulting from the combination of anionic and zwitterionic surfactants in the presence of salt. In more complex formulations the presence of other additives such as perfume and pearlescent agents can cause a deviation from a purely entangled worm-like micelle system. If this deviation persists in the absence of any additives, it can then be attributed to changes in the microstructure and the structuring efficiency of the surfactant system. The ability to achieve a fully entangled worm-like micelle system at low surfactant and low salt levels is highly desirable as it implies a highly efficient structuring system.
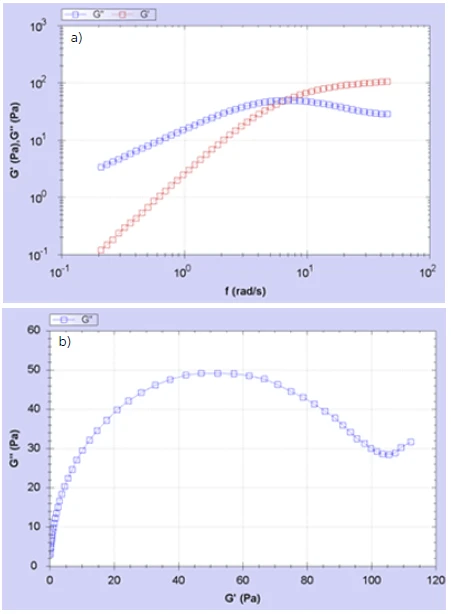
Table 2: Structural parameters extracted from measurement data using theory
The corresponding structural parameters extracted using theory are shown for this system in Table 2.
Conclusions
The properties of worm-like micelles (WLMs) represent a key research area in both academia and industry, since they are employed in a wide range of products and applications, many of which are critically dependent on their underlying microstructure. By combining rheological measurements with theoretical understanding it has been shown that it is possible to extract key microstructural parameters including the RelaxationWhen a constant strain is applied to a rubber compound, the force necessary to maintain that strain is not constant but decreases with time; this behavior is known as stress relaxation. The process responsible for stress relaxation can be physical or chemical, and under normal conditions, both will occur at the same time. relaxation time and hydrodynamic correlation length that are both characteristic and descriptive of the material and its rheological behavior.
Please note that a parallel plate geometry or a cylindrical geometry can also be used. The use of a solvent trap is also recommended for these tests since evaporation of solvent (e.g., water) around the edges of the measuring system can invalidate the test, particularly when working at higher temperatures.