Introduction
Hastelloy is a nickel-chromium-molybdenum-tungsten alloy with outstanding high-temperature stability as evidenced by high ductility and corrosion resistance. It has excellent resistance to StressStress is defined as a level of force applied on a sample with a well-defined cross section. (Stress = force/area). Samples having a circular or rectangular cross section can be compressed or stretched. Elastic materials like rubber can be stretched up to 5 to 10 times their original length.stress-corrosion cracking and to oxidizing atmospheres up to 1038°C. It is used in combustion gas desulfurization plants, chemical industry and incineration plants, etc.

Measurement Conditions and Results
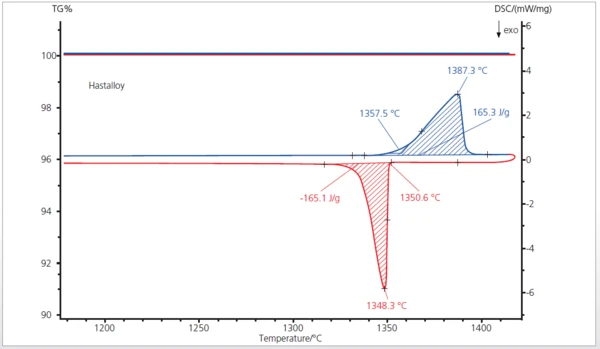
The STA measurement was carried out between room temperature and 1450°C. The DSC curve (blue) depicts the melting of a hastelloy sample (alloy 22) at 1358°C (extrapolated onset) with an enthalpy of 165 J/g. During cooling, CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization occurred at 1351°C (extrapolated endset) with nearly the same enthalpy change (red DSC curve). During heating and cooling, neither a mass loss nor an increase due to OxidationOxidation can describe different processes in the context of thermal analysis.oxidation was observed (TGA Signals)
Conclusion
Investigation of the melting and CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization behavior of metal alloys is possible with the STA 449 F5 Jupiter®. The vacuum-tight design allows for measurements under defined atmospheres improving the test results in terms of repeatability and accuracy. In addition, the risk of misinterpretation of the results due to OxidationOxidation can describe different processes in the context of thermal analysis.oxidation effects can be minimized.