11.05.2020 by Claire Strasser
Drug-Excipient Compatibility Check
Drug-excipient compatibility studies are an important part during the development of a new formulation. They ensure that no interaction occurs between drug and excipients that could affect the properties, stability, efficacy of the active ingredient. Thermal Analysis is used for rapid assessment of physicochemical interactions.
Generally, a drug product doesn't only contain the API (Active Pharmaceutical Ingredient), i.e., the substance fighting the sickness or pain, but also excipients, i.e., substances selected to facilitate administration of the dosage form, modulate the release of the API and stabilize it against degradation. Of course, the presence of the excipients must not affect the properties, stability, efficacy of the active ingredient. So-called drug-excipient compatibility studies are essential to reveal eventual interactions between the drug and excipients. An interaction can be underscored using spectroscopic and microscopic techniques, but also thermal methods, particularly thermal analysis, and more specifically differential scanning calorimetry (DSC) and thermogravimetry (TG). These two methods play an important role in compatibility screening and are frequently employed for rapid assessment of physicochemical interactions.
How does thermal analysis give first information about the drug-excipient compatibility?
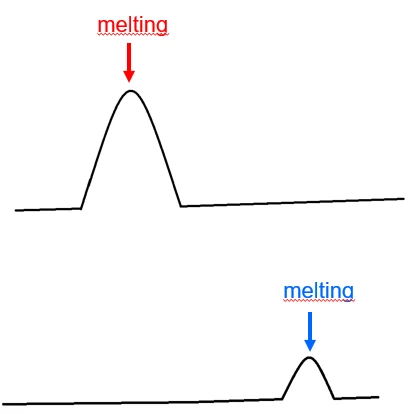
Let’s have a look at the DSC curves of 2 substances that interact – or not. For that, you carry out a DSC measurement on each component as well as on the mixture of both components (50/50 weight). Figure 1 displays the DSC curves of the API (active pharmaceutical ingredient) and excipient with melting peak.
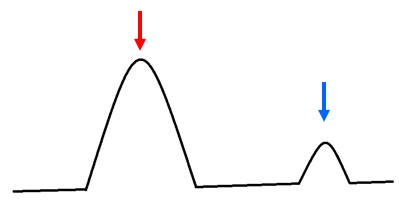
A resulting DSC curve that shows no interaction between the API and excipient (figure 2) indicates that the excipient is recommended for the formulation using the API. In this case, there is a compatibility between the API and the excipient; the DSC curve will continue to show the melting peaks of the two substances unaltered at the same temperature.
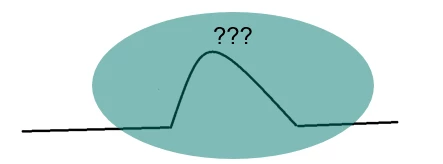
The occurrence of a new peak in the mixture, the disappearance of a peak, or a change in melting peak (shape, position, or enthalpy) indicates that there is an interaction between the two components (figure 3). However, this doesn’t necessarily mean that the drug and excipient are not compatible. Additional investigations would have to be carried out with other techniques (X-ray, spectroscopy, chromatography, etc.) to confirm incompatibility.
An example of compatibility study on diclofenac is given here. It shows how fast and easy thermal analysis detects drug/excipient interactions.