Introduction
Sporting goods and toys for kids or pets are often made of flexible plastics. Some examples are sensory chewing toys, action figurines, soft grip features as well as balls of any kind. A common polymer used is PVC (polyvinylchloride), which can be made softer and more flexible by adding plasticizers. These compounds are not covalently bonded to the polymer chain, and this is why they can evaporate or be rinsed out by saliva or sweat. Outgassing of plasticizers like phthalates can be harmful. In some cases, this can be even recognized by a bad smell.
The family of phthalates is known to cause a number of health risks. They act like hormones, and have been shown to cause liver damage, infertility, diabetes, cancer and more. Therefore, the European Union has banned a number of phthalates in products, which are in contact with food, in toys, in baby articles and medical supplies since 2007.
Decomposition Behavior and Identification of Plasticizers
Thermal analysis can help detect plasticizers in polymers. By means of TGA-FT-IR analysis, it is possible to analyze products regarding their plasticizer content and to identify the kind of plasticizer used.
In the following use case, the surface layer of different toy balls was cut in small pieces and measured with the PERSEUS® TG 209 F1 Libra® according to the measurement conditions in table 1.
Ball no. 1 exhibits several mass-loss steps during PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis, see figure 1. These mass-loss steps result from the evaporation of plasticizer or other organic additives and the PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis of the polymer in the temperature range between 200°C and 500°C. Decomposition of inorganic fillers was detected between 500°C and 700°C. The peaks in the DTG curve (mass-loss rate) represent the temperatures of the maximal mass-loss rates. The Gram Schmidt curve displays the overall IR intensities and behaves as a mirror image of the DTG curve and shows also maximum intensities during mass-loss steps. This proves the interaction of the evolved compounds with the IR beam.
Table 1: Measurement conditions
| Sample | Ball no. 1 | Ball no. 2 |
|---|---|---|
| Sample mass | 9.08 mg | 10.38 mg |
| Temperature program | RT to 850°C | |
| Heating rate | 10 K/min | |
| Gas atmosphere | Nitrogen | |
| Gas flow rate | 40 ml/min | |
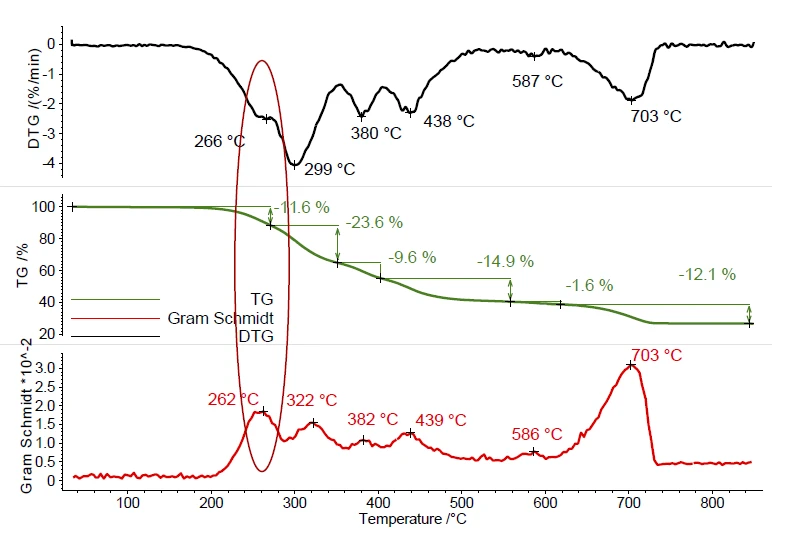
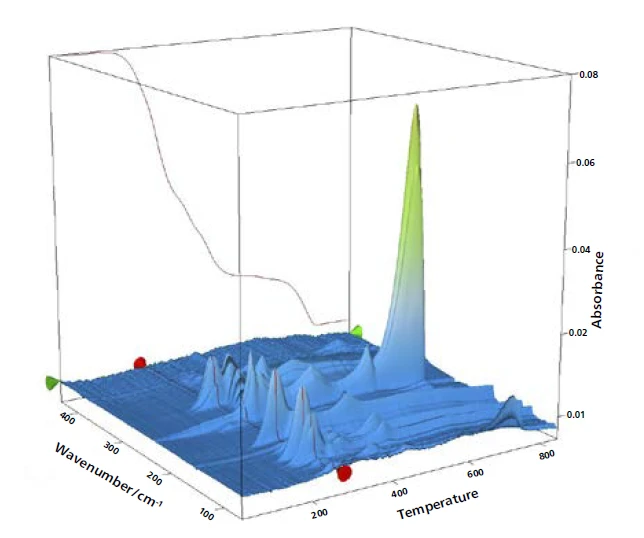
The complete IR data is shown in figure 2 in a temperature- and wavenumber-dependent 3D plot. The TGA curve is plotted in red at the back and shows the correlation of the mass loss to the increase in IR intensity. In this example, only the first mass-loss step is investigated more precisely. For detailed analysis of the plasticizer contained, a 2D FT-IR spectrum was extracted and compared to gas phase libraries to identify the evolved compounds. High similarity was found for the spectrum at 266°C to the library spectra of di-n-octylphthalate (DOP, blue) und bis(2-ethylhexyl)phthalate (DEHP, green). It can be assumed that a single compound or a mixture of different phthalates was released. However, this comparison clearly indicates that ball no. 1 contains harmful phthalates. As the following mass-loss step is slightly overlapping with the release of phthalates, some small amount of CO2 was also found by means of FT-IR at 266°C.
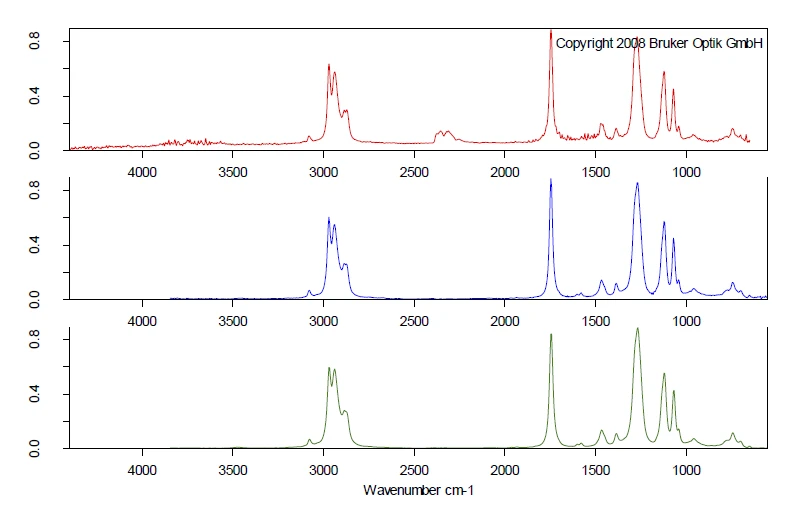
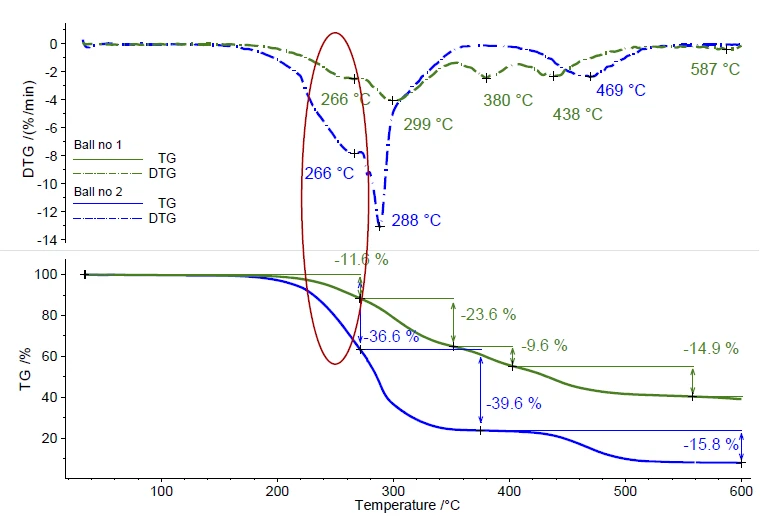
A second ball was investigated under the same measurement conditions. A comparison of both TGA measurements is shown in figure 4. A clear difference can be observed in the PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis behavior. However, also for ball no. 2, the first mass-loss step was detected in the temperature range between 200°C and 280°C, also with a peak in the DTG curve at 266°C. Only FT-IR can yield detailed information about the contained plasticizer.
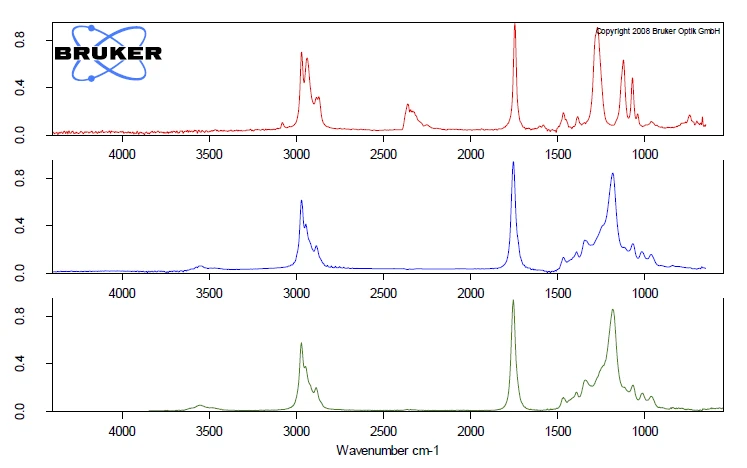
The comparison of the extracted FT-IR spectra for the two ball samples, both extracted at 266°C, show completely different VibrationA mechanic process of oscillation is called vibration. Vibration is a mechanical phenomenon whereby oscillations occur about an equilibrium point. In many cases, vibration is undesirable, wasting energy and creating unwanted sound. For example, the vibrational motions of engines, electric motors, or any mechanical device in operation are typically unwanted. Such vibrations could be caused by imbalances in the rotating parts, uneven friction, or the meshing of gear teeth. Careful designs usually minimize unwanted vibrations.vibration pattern, see figure 5. The comparison of the spectra at 266°C of ball no 2 (blue) to the gas phase library gives clear accordance with the spectrum of tributyl citrate (green). For ball no. 2, the toxic phthalate plasticizers were replaced by nontoxic citric ester, which also acts as a plasticizer.
Summary
Outgassing and Reazione di DecomposizioneLa decomposizione è la reazione indotta termicamente con cui una sostanza chimica genera prodotti solidi e/o gassosi. decomposition processes of polymers can be investigated by thermal analysis. Thermogravimetry indicates the release of gases already below 300°C. Only evolved gas analysis like FT-IR can identify the gases released. In this example, it was possible to identify the different plasticizers used and therefore, to distinguish between toxic and non-toxic additives. The PERSEUS® TG 209 F1 Libra® is perfectly suited to solve this task.