Wprowadzenie
W farmacji nie ma prawie żadnego aktywnego składnika, o którym napisano by więcej niż o kwasie acetylosalicylowym (lub w skrócie ASA; w krajach anglojęzycznych nawet nazwa marki Aspirin™ jest często używana jako synonim). Historia jego sukcesu rozpoczęła się pod koniec XIX wieku, kiedy dr Felix Hoffmann po raz pierwszy zsyntetyzował tę substancję w laboratoriach BAYER bez zanieczyszczeń. Obecnie jest to nadal jeden z najpopularniejszych farmaceutyków stosowanych w szerokim zakresie terapeutycznym. Należy do grupy niesteroidowych leków przeciwzapalnych (NLPZ) i jest wskazany w leczeniu bólu, gorączki i stanów zapalnych. Ponadto jest stosowany w zapobieganiu nawrotom zawału serca lub udaru mózgu u pacjentów z grupy wysokiego ryzyka. W 1977 roku ASA został dodany jako lek przeciwbólowy do "listy podstawowych leków" WHO (Światowej Organizacji Zdrowia). [1]
Jest to jedna z czterech not aplikacyjnych, które bardziej szczegółowo badają zachowanie termiczne kwasu acetylosalicylowego: Rozkład w różnych atmosferach gazowych, kinetyka rozkładu i powstające gatunki gazów. [2, 3, 4]
Tabela 1: Parametry pomiarowe STA
| Parametr | Kwas acetylosalicylowy |
| Masa próbki | 4.96 mg |
| Atmosfera | Hel |
| Tygiel | Al2O3, 85 μl, otwarty |
| Program temperatury | RT do 50 °C, 10 K/min |
| Natężenie przepływu | 100 ml/min |
| Uchwyt próbki | TGA, typ S |
Wyniki i dyskusja
W celu zbadania rozkładu termicznego kwasu acetylosalicylowego przeprowadzono pomiary termograwimetryczne (TGA) za pomocą urządzenia NETZSCH STA 449 F3 Jupiter® , sprzężonego z systemem GC-MS (chromatograf gazowy Agilent 8890 i Agilent 5975 MSD). Jako atmosferę gazu oczyszczającego zastosowano gazy obojętne, takie jak hel. Szczegółowe informacje na temat warunków pomiaru podsumowano w tabeli 1.
PirolizaPiroliza to termiczny rozkład związków organicznych w atmosferze obojętnej.Piroliza kwasu acetylosalicylowego wykazuje dwa etapy utraty masy (patrz rysunek 1). Pierwszy ubytek masy wynoszący 66,4% jest związany ze szczytem szybkości ubytku masy (DTG) w temperaturze 170°C. Drugi etap utraty masy wynosi 33,4% z pikiem na krzywej DTG w temperaturze 327°C.
Aby zapewnić wgląd w produkty pirolizy, zastosowano sprzężenie TGA-GC-MS w celu oddzielenia złożonej mieszaniny gazów i identyfikacji różnych składników. Parametry pomiarowe GC-MS opisano w tabeli 2.
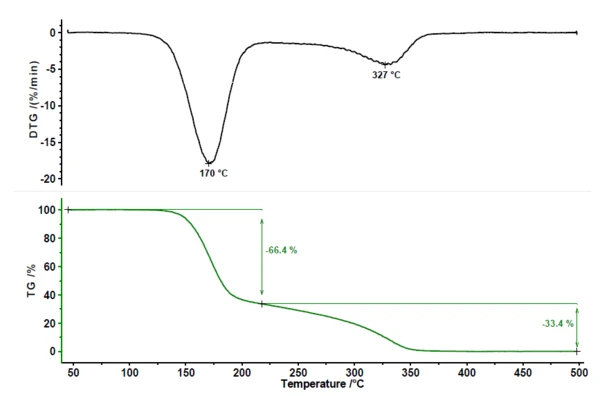
Tabela 2: Parametry pomiarowe GC-MS
| Parametr | Tryb pułapkowania kriogenicznego |
| Kolumna | Agilent HP-5ms |
| Długość kolumny | 30 m |
| Średnica kolumny | 0.25 mm |
| Pułapka kriogeniczna | -50°C, 45 min |
| Temperatura kolumny | 40°C, izoterma, 48 min 40°C do 300°C, 15 K/min |
| Atmosfera gazowa | On |
| Przepływ w kolumnie (split) | 2 ml/min (5:1) |
| Zawór | Co 1 min |
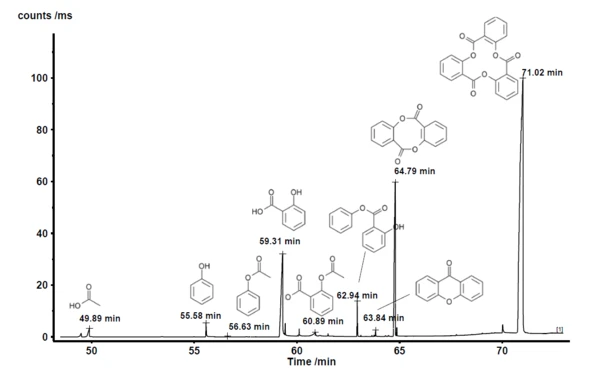
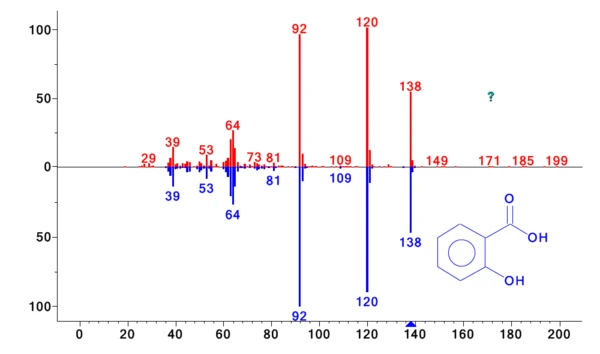
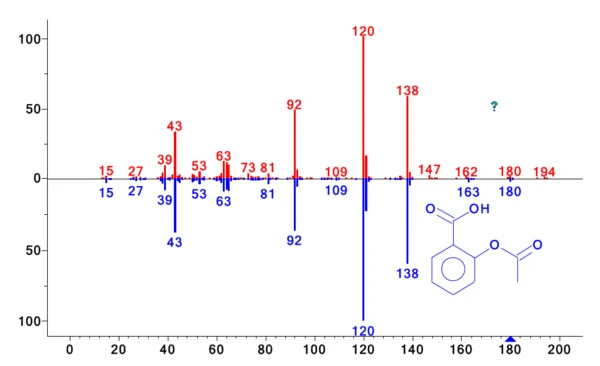
Próbki uwalnianych gazów były pobierane co minutę w pułapce kriogenicznej. Po zakończeniu pomiaru termograwimetrycznego, krio pułapka została podgrzana z -50°C do 300°C z szybkością 300 K/min, aby odparować skondensowane związki i pozwolić im oddzielić się na kolumnie GC (która była podgrzewana z szybkością 15 K/min). Metoda ta zwiększa stężenie produktów ubocznych i umożliwia doskonałą separację. Wynikowy całkowity prąd jonowy przedstawiono na rysunku 2. Porównanie wykrytych widm MS dla każdego piku z NIST library daje szereg związków o doskonałej jakości trafienia. Przykłady identyfikacji pokazano dla pików o czasie retencji 59,31 min i 60,89 min na rysunkach 3 i 4. Oprócz kwasu octowego, fenolu, kwasu salicylowego i kwasu acetylosalicylowego znaleziono również cykliczne oligomery kwasu 2-hydroksybenzoesowego, jak podano w literaturze. Analiza ta ujawnia, żeReakcja rozkładuReakcja rozkładu to wywołana termicznie reakcja związku chemicznego tworząca produkty stałe i/lub gazowe. rozkład i OdparowanieOdparowanie pierwiastka lub związku jest przejściem fazowym z fazy ciekłej do pary. Istnieją dwa rodzaje parowania: parowanie i wrzenie.odparowanie zachodzą jednocześnie, a ponadto wyjaśnia, dlaczego dwa etapy utraty masy nie są rozdzielone.
Library Search
| Czas retencji [min] | Nazwa | Jakość trafienia |
| 49.89 | Kwas octowy | 91 |
| 55.58 | Fenol | 96 |
| 56.63 | Ester fenylowy kwasu octowego | 90 |
| 59.31 | kwas 2-hydroksybenzoesowy (= kwas salicylowy) | 97 |
| 60.89 | Kwas acetylosalicylowy | 81 |
| 62.94 | Salicylan fenylu | 95 |
| 63.84 | Ksanton | 97 |
| 64.79 | 6H,12H-Dibenzo[b,f][1,5]dioxocin-6,12-dione (Dimer kwasu 2-hydroksybenzoesowego) | 64 |
| 71.02 | 2,10,18-Trioxatetracyclo[18.4.0.0(4,9).0(12,17)] tetracosa-1(24),4,6,8,12,14,16,20,22-nonaene-3,11, 19-trion (trimer kwasu 2-hydroksybenzoesowego) | 90 |
Wnioski
Połączenie termograwimetrii i GC-MS (chromatografii gazowej / spektrometrii mas) jest potężną techniką umożliwiającą uzyskanie głębokiego wglądu w procesy rozkładu termicznego i uwalniane gazy. Rozkład termiczny kwasu acetylosalicylowego w atmosferze helu skutkuje powstaniem złożonej mieszaniny gazowej składającej się z co najmniej dziewięciu różnych uwalnianych związków. Poprzednie badania za pomocą TGA-FT-IR (spektroskopia w podczerwieni z transformacją Fouriera sprzężona z termobalansem) wykazały, że pierwszy etap utraty masy uwalnia kwas octowy i kwas salicylowy, podczas gdy drugi etap utraty masy jest wynikiem złożonej reakcji rozkładu. Możliwości GC-MS zaczynają się tam, gdzie FT-IR osiąga swoje ograniczenia i zapewnia znacznie głębszy wgląd w mieszaniny jednocześnie uwalnianych gazów. TGA-GCMS jest w stanie zarówno je rozdzielić, jak i zidentyfikować.