
29.07.2020 by Dr. Gabriele Kaiser
Investigating the Purity of Substances by Means of DSC
Differential Scanning Calorimetry (DSC) offers a quick and easy way to determine the purity of crystalline substances. Only one measurement with a few milligrams of sample is needed. Read here how it works!
The evaluation usually runs automatically while the calculation is based on the Van´t Hoff equation which takes the melting point depression due to the presence of impurities into account. Application of the method requires that the impurities dissolve in the melt and are insoluble in the solid phase.
Methylparaben ‒ a Widely Used Antimicrobial Preservative
Methylparaben (or Nipagin; IUPAC name: Methyl 4-hydroxybenzoate) is a frequent ingredient of cosmetics, food products (E 218) as well as oral and topical pharmaceutical formulations. It is a white crystalline powder with a Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature of 125°C to 128°C as specified in the corresponding methylparaben monograph (as Identification B). The picture displays microcrystals of methylparaben.
Purity Determination
Figure 1 shows the DSC result of a measurement on methylparaben performed at 0.7 K/min in aluminum crucibles in a nitrogen atmosphere. In ASTM E928 (1) which describes the purity determination by DSC in more detail, small heating rates and small sample masses are recommended for such experiments:
- Heating rates between 0.3 K/min and 0.7 K/min
- Sample masses between 1 mg and 3 mg
Additionally, minimum 200 points should be acquired in the melt region.
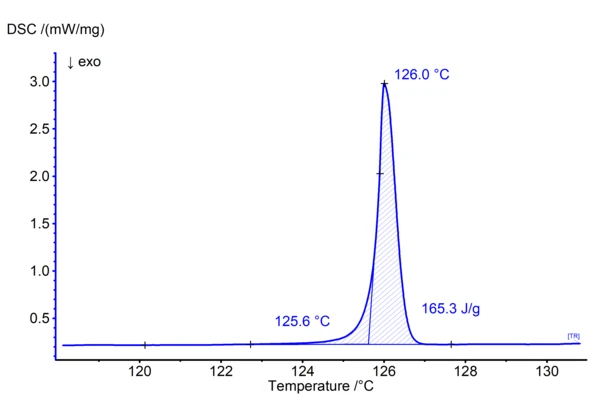
The present measurement meets all requirements for purity determination:
- Methylparaben is a crystalline substance
- The polymorphic form does not change during heating (present is the monoclinic form I)
- There is a clear melting effect without any disturbance
- The mass loss occurring during melting is smaller than 1% (threshold given in ASTM E928)
The resulting Van´t Hoff plot (Fig. 2) displays the reciprocal value of the partial area (1/F as x-axis) along with the temperature at which that amount of melting is observed (y-axis). The non-linear data is directly taken from the measurement curve; the line represents the data after linearization. The total amount of impurities is derived from the slope of the linear curve; in the current case, it accounts for 0.14 mol%. Thus, the purity of the methylparaben used is 99.86 mol%. The intersection point of the linear curve with the y-axis at 1/F = 0 yields the theoretical Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature of the 100% pure sample, here 126.063 °C. The Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature of the measured substance is related to the temperature value which corresponds to 1/F = 1. More information about the Van´t Hoff plot can be found here.
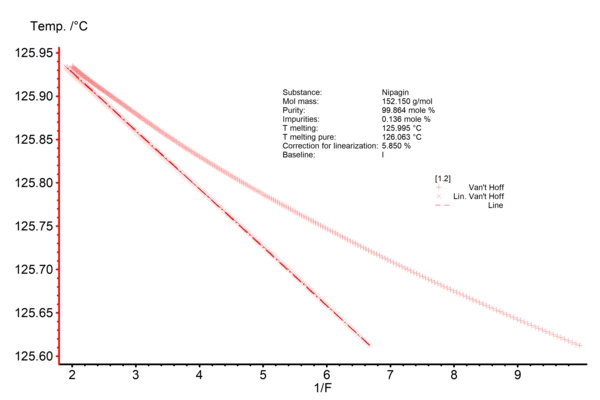
Fig. 2: Purity determination of methylparaben, Van´t Hoff plot Displayed are both the non-linear (+) and linearized data (x) along with the table of results; Tmelting and Tmelting pure are derived by means of extrapolation of the linearized data Literature: (1) ASTM E928, Standard Test Method for Purity by Differential Scanning Calorimetry