Introduction
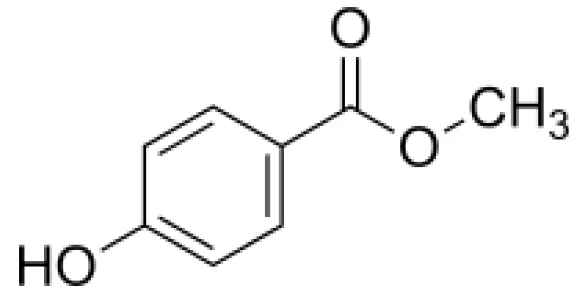
Nipagin is a white powder with the chemical name methyl 4-hydroxybenzoate (figure 1). It is used a preservative in cosmetics, medications, and in foods under the name E218 [1, 2].
Product degradation and Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition investigations are fundamental tests for any pharmaceutical or similar products applied in the food and cosmetic industries. The experiments expose the material and other substances within the product to an external StressStress is defined as a level of force applied on a sample with a well-defined cross section. (Stress = force/area). Samples having a circular or rectangular cross section can be compressed or stretched. Elastic materials like rubber can be stretched up to 5 to 10 times their original length.stress to assess the stability of the constituents or formulation. External StressStress is defined as a level of force applied on a sample with a well-defined cross section. (Stress = force/area). Samples having a circular or rectangular cross section can be compressed or stretched. Elastic materials like rubber can be stretched up to 5 to 10 times their original length.stress can be, for example, pH (acid-base balance), light, moisture, temperature, etc. For investigations which are based on thermal stresses, coupling of the techniques TGA and FT-IR is a suitable method to achieve fast characterization of the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition, degradation and Thermal StabilityA material is thermally stable if it does not decompose under the influence of temperature. One way to determine the thermal stability of a substance is to use a TGA (thermogravimetric analyzer). thermal stability behavior.
In the following, the thermal behavior of a Nipagin sample was characterized by means of TGA-FT-IR.
Test Conditions
The measurement was carried out on Nipagin (9.22 mg) weighed in an open aluminum oxide crucible. The sample was placed in the TG 209 F1 Libra® and heated up to 600°C at 10 K/min under a dynamic nitrogen atmosphere. The gases evolved during heating were directly transferred into the gas cell of the FT-IR spectrometer manufactured by Bruker Optics.
Test Results
Figure 2 depicts the mass variations of Nipagin during heating (green curve). The mass loss of 100% indicates the complete Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition between approx. 125°C and 230°C. The Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point of Nipagin is located at 125.2°C [2]. This means that the substance decomposes immediately after its melting.
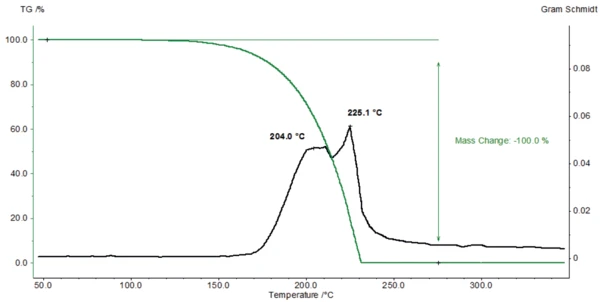
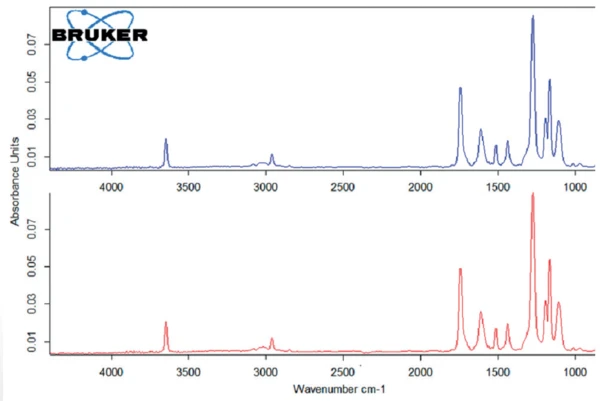
The curve known as Gram Schmidt (black) indicates the concentration of the evolved substances detected by the FT-IR as a function of time. Figure 3 shows the FT-IR-spectra of the products released at 206°C and 227°C, which are identical.
The band at 3648 cm-1 is typical for O-H bonds. The bands with lower intensities at 2800 cm-1 and 3100 cm-1 are characteristic for C-H vibrations of alkanes (< 3000 cm-1) and alkenes (> 3000 cm-1). The band at 1745 cm-1 indicates C=O vibrations. The range between 1450 cm-1 and 1625 cm-1 can be attributed to aromatic skeletons.
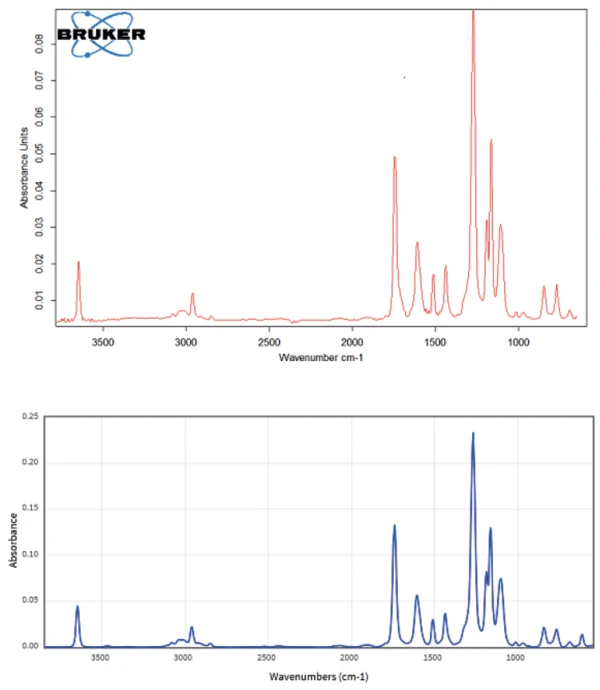
A comparison of the measured FT-IR spectrum of the products released at 227°C with the literature FT-IR spectrum of Nipagin in figure 4 reveals that the two spectra are practically identical. This means that Nipagin does not decompose, but evaporates during heating in an inert atmosphere.
Conclusion
TGA-FT-IR measurements allow for investigations of the Thermal StabilityA material is thermally stable if it does not decompose under the influence of temperature. One way to determine the thermal stability of a substance is to use a TGA (thermogravimetric analyzer). thermal stability of materials such as pharmaceuticals, cosmetics and foods. In the case of Nipagin, it was observed that the mass loss was not due to Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of the sample, as would have been deduced from the TGA measurement alone, but to evaporation. The TGA-FT-IR spectrum at the highest Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition rate clearly shows the Nipagin fingerprint confirmed by literature data.
With the TGA-FT-IR coupling, a tool has been developed which easily qualifies the mass losses observed in the TGA and thus allows one to draw conclusions as to whether a material decomposes, degrades, or evaporates under thermal influence.