Introduction
Definded heating and cooling rates are important parameters for DSC measurements. International standards recommend a heating rate of 10 K/min or 20 K/min (ISO 11357, DIN 53765, ASTM E793, ASTM E794) when striving for thermodynamic equilibrium. In contrast, the objective of quality control and assurance in polymer processing is to obtain meaningful measurement results faster by means of higher heating rates (e.g., 40 K/min). The primary aim is to compare a current measurement on a rejected part to a control part.
Influence of Heating and Cooling Rates Using the Example of PBT
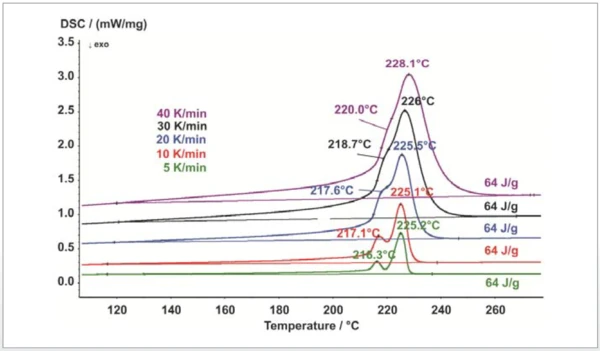
Figure 1 shows the melting behavior of polybutylene terephthalate (PBT) at an increasing heating rate. The measurements were carried out with the DSC 204 F1 Phoenix® in a nitrogen atmosphere. The relatively high heating rate of 40 K/min for semi-crystalline PBT no longer shows the typical melting of the ß-phase seen in smaller crystallites, but rather only the main melting peak (here at 228°C). If attempting material identifi cation, it could be incorrectly assumed here that the material in question is polyamide (PA 6). The lower heating rate of 10 K/min already shows the ß-phase clearly separated from the main peak at 217°C; this is typical for PBT and does not occur for PA6.
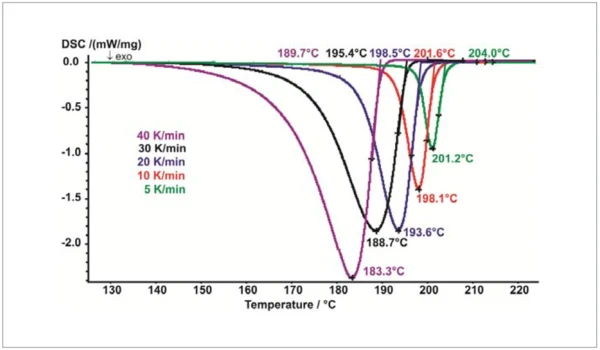
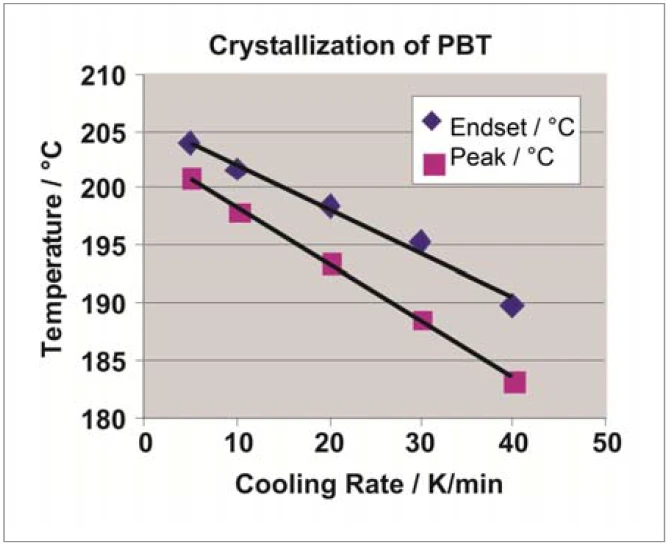
Controlled cooling from the melt carried out with the intracooler yields the CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization behavior of PBT (figure 2). As the cooling rate increases, both the beginning of solidification (extrapolated end, viewing direction from right to left) and the CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization peak temperature shift to lower values (figure 3). As the cooling rate increases, the CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization peak not only gets larger but also extends across a broader temperature range. Although considerably higher cooling rates are employed in injection molding, DSC yields important information as to when or at which temperature the part can be demolded from the tool safely and without danger of distortion.
Summary
The operator dutifully carries out temperature calibration at higher heating rates and records a shift of the melting peak temperature at higher values, but is then often surprised that the DSC measurement on the real polymer sample does not deliver the desired result. The high heating rate causes thermal eff ects to be displaced; individual peaks or melting phases can no longer be reliably separated. The cooling rate also influences the CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization behavior. Fast cooling rates cause a delay inCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization, but serve for optimizing production processes.