Introduction
Solvent evaporation or sample drying can occur in a variety of samples during long-term rheological testing or when testing at elevated temperatures. This is particularly problematic for volatile samples containing organic solvents but even aqueous-based materials such as ketchup or pastes have been seen to ‘dry out’ quickly when exposed to the atmosphere.
Parallel plate or Cone-Plate measuring systems are most susceptible to evaporation and drying since the materials at the plate or cone edge are directly exposed. The issue is further exaggerated by the fact that the measured or applied torque (M) varies with the radius (r) to the power of four (r4) for a plate and to the power of three (r3) for a cone, when measuring a Newtonian material. Consequently, any change in sample properties at the edge caused by crust formation or loss of sample will have a significant impact on the measurement result. Problems may be encountered with concentric cylinder systems also, albeit to a lesser extent.
To overcome such issues, it is recommended to use a solvent trap as illustrated in Figure 1 below. The Kinexus Passive solvent trap system illustrated contains an outer thermal cover (Nylon 66 Glass filled 30%), which is the same material as the standard sample covers that are included with the standard plate and cylinder cartridges on the Kinexus. The inner material is made of stainless steel, and has an overlapping feature over the entire seam to give a tight seal. An additional feature is an included purge gas option that can be used to purge gas or vapor into the chamber if required. Inside, there are two reservoirs for solvent, one located around the geometry shaft and the other a circular channel located around the circumference of the lower plate. The solvent ring and the plate channel are filled with the sample solvent. When the system is sealed, the vapour creates a saturated system reducing the evaporation of the sample solvent.
The solvent trap is compatible with both plate and cylinder cartridges.
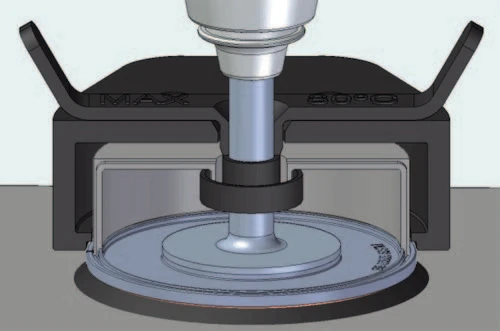
This application note highlights the problem of solvent evaporation in rheological measurements and shows how it can be overcome by using a solvent trap during rheological testing.
Experimental
- A Ketchup sample was measured with and without a solvent trap system in this study to determine its effectiveness at preventing sample drying.
- Rotational rheometer measurements were made using a Kinexus rheometer with a Peltier plate cartridge and a 40 mm roughened plate-plate measuring system, utilizing standard pre-configured sequences in rSpace software.
- A standard loading sequence was used to ensure that the samples were subjected to a consistent and controllable loading protocol.
- All rheology measurements were performed at 25°C.
- A single frequency StrainStrain describes a deformation of a material, which is loaded mechanically by an external force or stress. Rubber compounds show creep properties, if a static load is applied.strain controlled experiment was performed within the Linear Viscoelastic Region (LVER)In the LVER, applied stresses are insufficient to cause structural breakdown (yielding) of the structure and hence important micro-structural properties are being measured.linear viscoelastic region over times ranging from 20 to 65 hours, with and without the Kinexus solvent trap employed.
- The solvent ring and lower plate channel were filled with the sample solvent; in this case water.
Results and Discussion
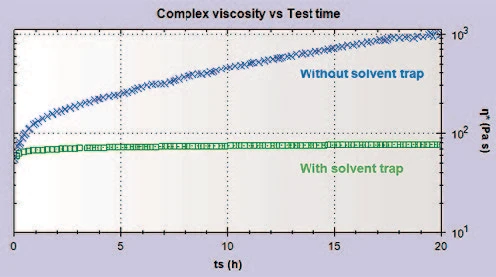
Figure 2 shows a plot of the complex viscosity with time for a Ketchup sample, with and without a solvent trap present, over a testing period of 20 hours. Without the solvent trap present, the complex viscosity increases with time; this shows that the sample is drying out with exposure to the atmosphere. With the solvent trap employed, the complex viscosity is much more consistent, suggesting that extended testing could be performed on this sample without concern that the sample is changing.
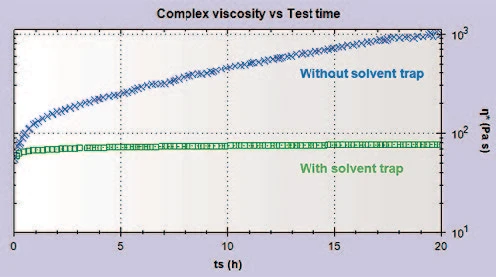
To evaluate how long the measurement could be extended with the solvent trap employed, measurements were repeated up to 65 hours of testing (Figure 3). A small increase in the complex viscosity was initially observed; this was attributed to thixotropic recovery after loading, since Ketchup is a thixotropic material. After this initial recovery period, however, the complex viscosity remained relatively constant for the 65 hours of testing. The final viscosity measured after 65 hours of testing with the solvent trap employed was attained after just 25 minutes with no solvent trap present illustrating how important and effective it is at preventing evaporation.
Conclusion
Using a solvent trap can effectively prevent sample drying/ solvent loss for a long period of time during extended testing. This is essential to ensure that only rheological changes are being measured and not artifacts resulting from solvent loss or drying of the sample at the air/sample interface.