Introduction
One‘s primary interest when investigating polymers with a thermobalance is to obtain information about mass changes as a function of temperature. This can yield information on possible additives and fillers as well as the polymer content. Changing from an inert to an oxidizing atmosphere allows for the targeted combustion of Added Carbon BlackCarbon black acts as a reinforcing filler in tires and other rubber products. In other materials such as plastics, paints, and inks, carbon black is used as a color pigment or as filler to achieve electric conductivity.added carbon black or Pyrolytic CarbonPyrolytic carbon is carbon which is generated by the pyrolysis of organic matter in an oxygen-free atmosphere. pyrolytic carbon, while the residual mass loss yields information about the type and amount of fillers employed and the ash conent. It is not possible, however, to fully describe the sample‘s properties, or to identify an unknown polymer, because certain information is missing; specifically information about the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature. This is because – in contrast with a DSC or DTA apparatus – instruments for thermogravimetric measurements generally have only one sample position in the sample chamber. The TG 209 F1 Libra® sample holder – which can accommodate a single sample crucible – is depicted in figure 1.
This means – in contrast with instruments having two measuring positions in the sample chamber (such as a DSC and DTA) – a measured differential signal cannot be evaluated with this instrument. Thermal effects such as evaluation of the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature cannot be recorded. This shortcoming can be rectified, however, with the help of the c-DTA signal. This considerably increases the value of the TGA apparatus by yielding DTA-like information in addition to just thermogravimetric information.

Measurement Conditions for the Investigations Shown in Figure 3
| Sample | PE | PP | PA6 |
|---|---|---|---|
| Sample mass | 7.3 mg | 10.47 mg | 8.77 mg |
| Crucible | Al2O3 | Al2O3 | Al2O3 |
| Atmosphere | Nitrogen | Nitrogen | Nitrogen |
| Gas flow rate | 40 ml/min | 40 ml/min | 40 ml/min |
| Heating rate | 20 K/min | 20 K/min | 20 K/min |
How the c-DTA Works
The c-DTA evaluation compares the measured signal of the sample temperature with the preset nominal value; i.e., with the calculated temperature-time program. At the point in time that a caloric transition takes place in the sample, the measured sample temperature deviates from what the linear course had been prior to the transition. If the sample melts (endothermal effect), for example, the applied energy is needed for the melting process and therefore does not immediately cause an increase in temperature, so the sample temperature remains behind the programmed linear heating rate. The schematic in figure 2 compares the measured temperature signal to the calculated nominal value of the temperature program.
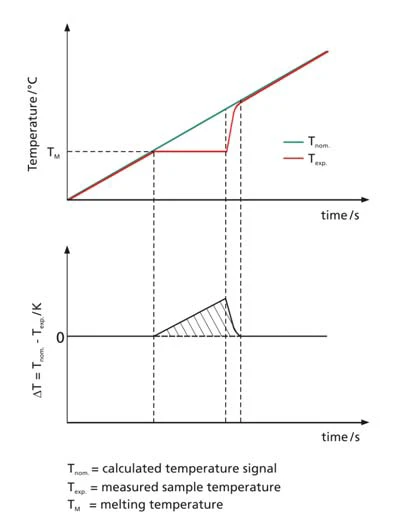
The resulting differential signal is called the calculated DTA signal (c-DTA). For the reasons described above, it does not have the quality of a measured DSC signal, but it can still provide valuable clues for identifying unknown samples, as is shown below. A second important application is the ability to determine the melting temperatures of standard calibration substances via the c-DTA signal. This allows for temperature calibration using established melting standards, as can be done with measurement instruments having a twinned design (such as DSC).
Results
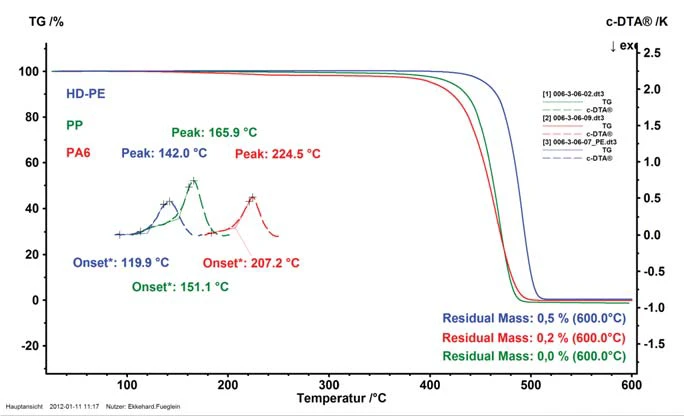
Figure 3 compares the analytical results for three common thermoplastics, polyethylene (HD-PE), polypropylene (PP) and polyamide 6 (PA6).
In addition to the thermogravimetric information, the c-DTA signals (dashed lines) are presented for each sample in the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature range. With the extrapolated onset and the peak temperature, they identify the melting range of the sample. A comparison of the materials HD-PE, PP and PA6 shows clearly that additional information for helping to identify unknown samples can be thereby obtained.
Besides determining the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point of the samples under investigation, c-DTA evaluation also offers an elegant temperature calibration method. While investigation of the melting behavior would simply be impossible without the c-DTA evaluation, this function also allows for determination of the melting temperatures of common calibration materials. These results are used in calculating a temperature polynomial for temperature calibration and ensure reliable temperature evaluation for all subsequent investigations.
Presented in figure 4 is a summary of the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature determination for different calibration materials by means of the c-DTA method.
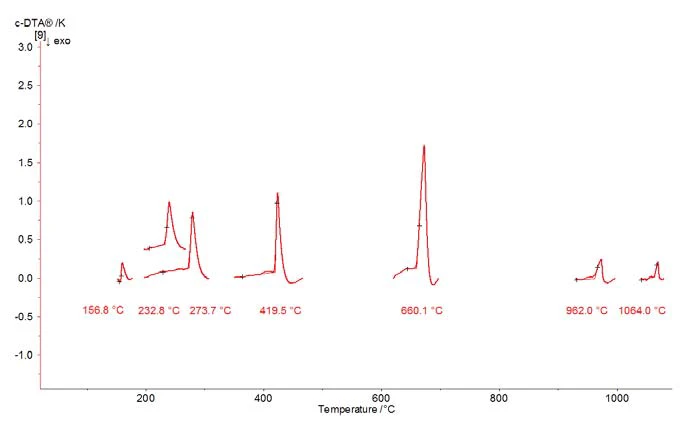
For the temperature calibration of a thermobalance, the calibration substances selected should span the temperature range from 25°C to 1100°C. For calculation of the polynomial, a minimum of three substances are required.
Table 1: Summary of the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point determination for seven calibration substances
| Sample | Indium | Tin | Bismuth | Zinc | Aluminum | Silver | Gold |
|---|---|---|---|---|---|---|---|
| Sample mass/mg | 4.689 | 5.268 | 8.392 | 6.159 | 5.425 | 5.078 | 4.564 |
| Tnom./°C | 156.6 | 231.9 | 271.4 | 419.5 | 660.3 | 961.8 | 1064.2 |
| Texp./°C | 156.8 | 232.8 | 273.7 | 419.6 | 660.1 | 962.0 | 1064.0 |