Functional principle of a heat-flux DSC
A DSC measuring cell consists of a furnace and an integrated sensor with designated positions for the sample and reference pans.
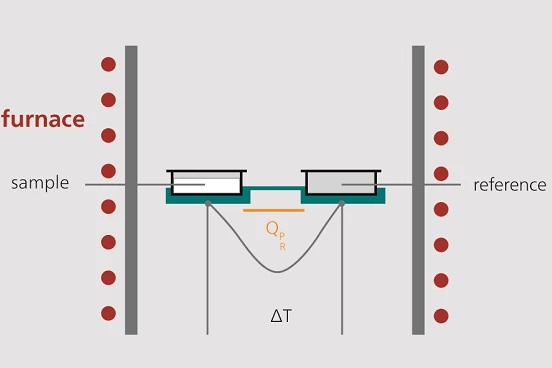
The sensor areas are connected to thermocouples or may even be part of the thermocouple. This allows for recording both the temperature difference between the sample and reference side (DSC signal) and the absolute temperature of the sample or reference side.
Due to the heat capacity (cp) of the sample, the reference side (usually an empty pan) generally heats faster than the sample side during heating of the DSC measuring cell; i.e., the reference temperature (TR, green) increases a bit faster than the sample temperature (TP, red). The two curves exhibit parallel behavior during heating at a constant heating rate – until a sample reaction occurs. In the case shown here, the sample starts to melt at t1. The temperature of the sample does not change during melting; the temperature of the reference side, however, remains unaffected and continues exhibiting a linear increase. When melting is completed, the sample temperature also begins to increase again and, beginning with the point in time t2, again exhibits a linear increase.
The differential signal (ΔT) of the two temperature curves is presented in the lower part of the image. In the middle section of the curve, calculation of the differences generates a peak (blue) representing the EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic melting process. Depending on whether the reference temperature was subtracted from the sample temperature or vice versa during this calculation, the generated peak may point upward or downward in the graphs. The peak area is correlated with the heat content of the transition (enthalpy in J/g).
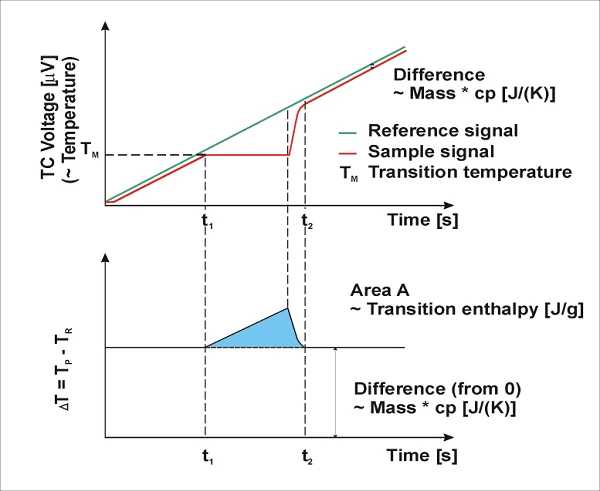
DIN 51007 and ISO 11357-1 recommend the portrayal of EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic processes with upward ordinate amplitude. In, for example, ASTM E793 and E794, downward application of the EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic direction is suggested. This is why the NETZSCH Proteus® software allows for the direction of application for EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic and ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic processes to be selected.






