Terms and Origin
Chocolate was known to humankind as far back as the Aztecs, but in the form of a cocoa-containing beverage. The term “chocolate“ is derived from the Aztec word Xocolatl meaning bitter water or cocoa water. The drink was made from the seeds of the cacao plant and cold water and was considered to be inebriating. In the Aztec world, it was reserved for adult men of noble descent and not considered suitable for women and children. The Aztec King, Montezuma, reportedly drank large quantities of cocoa. Under his reign, cocoa beans were also used as form of currency.
In 1528, the Spanish conquisitadors of the Hernán Cortés‘ era brought cocoa to Europe; the beverage was first tasted at the Spanish court in 1544. In 1673, the Dutchman Jantz von Huesden served chocolate to the public for the first time in Bremen. However, it was not until the 18th and 19th centuries that cocoa beans were treated there in larger quantities. Since they were very expensive, only the rich nobility could afford them.
In 1804, A. Miehte founded the Halloren Chocolate Factory, the oldest chocolate factory in Germany, in the town of Halle an der Saale.
The first Swiss chocolate factory was founded by François-Louis Cailler in 1819 in Vevey, followed by Philippe Suchard (1824), Jean Tobler (1830), Rudolf Sprüngli (1845) and Daniel Peter and Henri Nestlé (1875). The conching process, which largely contributed to the excellent reputation of Swiss Chocolate, dates back to Rudolphe Lindt.
Cocoa Nibs, Cocoa Mass, Cocoa Butter and Cocoa Powder
The botanical name of the cacao tree, Theobroma cacao, is derived from the Greek (theos: “God“; broma: “food“). This name expresses the high appreciation for this plant. Theobroma cacao is a cauliflorous plant, and thus develops both its blossoms and later its fruits on the already lignified trunk (figure 1).

The 15 - 20 cm long yellow fruits weigh approximately half a kilo and contain 30 to 60 white seed beans. Upon harvest, these are detached fermented and dried. During fermentation, which takes approximately 10 days, many bitter substances are decomposed and the cocoa beans develop their characteristic flavor and color.

Figure 2 shows the fermented, unpeeled beans. It is typically in this state that the beans are shipped to other countries, where they are then processed into chocolate. Cocoa mass – which is important for the production of chocolate – is yielded when the beans are broken up; this then processed into cocoa powder and cocoa butter.
Cocoa mass is actually the term for the cocoa nibs remaining after the beans are dried and the shell parts removed. When these nibs are ground, the fat contained with them – the cocoa butter – fl ows out and binds the nibs into a viscous, dark brown mass. When this cocoa mass is pressed, the cocoa butter fl ows out and the pressed cake can be ground into cocoa powder. Depending upon the residual fat content, this powder is designated as strongly de-oiled (approx. 11% to 12% fat) or lightly de-oiled (approx. 20% to 22% fat).
Ingredients and Effect
Besides the relatively high fat content (54% cocoa butter), cocoa also contains a few substances known to have a moodenhancing effect. These are serotonin, dopamine und theobromine (3.7-dimethyl xanthine, C7H8N4O2), a substance from the methylxanthine class very similar to caffeine. Although only small concentrations of these ingredients are contained in cocoa, they must be the reason behind the common conception that “chocolate makes you happy“. The health aspects of cocoa consumption have not yet been conclusively determined and are still the subject of current research work. However, health-promoting effects have been confi rmed in numerous separate studies, especially for chocolate with a high cocoa content (> 50%). These positive effects include the reduction of deposits in blood vessels, a lowering of blood pressure and LDL cholesterol levels, and improved skin functionality and general physical performance.
Figure 3 shows an assortment of chocolate bars with differing levels of cocoa content.

Polymorphism of Cocoa Butter
Chemically, cocoa butter is mainly composed of triglycerides from different fatty acids, primarily palmitic acid, stearic acid, oleic acid and linoleic acid. Due to the pronounced PolymorphismPolymorphism is the ability of a solid material to form different crystalline structures (synonyms: forms, modifications).polymorphism of cocoa butter, it is known to have six crystal structures which melt in the temperature range between 17°C and 36°C. For the production of chocolate, it is particularly important that the V polymorph – the so-called “ß-modifi cation“ – is formed during solidifi cation of the liquid chocolate mass. This is achieved by a special heat treatment called “tempering“. During tempering, the chocolate mass is subjected to a defi ned cooling and is then reheated in order to re-melt undesired low-melting crystals. It is diffi cult to fi nd the correct temperature here since the formation of crystallization nuclei in cocoa butter takes place very slowely; i.e., the crystallization process is very slow and the chocolate mass can be highly overcooled before crystallization becomes noticeable. How-ever, in heating instances where the low-melting crystal forms have already liquiefi ed, but suffi cient amounts of highmelting crystals – which are the most stable ß-modification in thermo-dynamic terms – still remain, then these end up serving as crystallization nuclei for the subsequent cooling. Therefore, during re-cooling, it is almost exclusively the desired ß-modification which is formed.
This process can easily be reproduced and analyzed by means of Differential Scanning Calorimetry (DSC). The melting behavior of a certain chocolate (with 60% cocoa content) is presented in figure 4. For the ß-modification which is targeted during the production of chocolate, melting begins slowly at around 25°C and reaches its peak maximum in the first heating (red) at 33.2°C. During cooling (blue), the start of crystallization at 22.7°C can be detected as an extrapolated endset.
However, part of the melt can be overcooled to 15°C before these portions begin to crystallize. At a cooling rate of 5 K/min, it takes this sample until approximately -5°C to fi nish crystallization. It can already be seen from the peak form of the cooling curve that – in contrast with the preceding industrial production situation – multiple modifi cations of the cocoa butter which melt at lower temperatures have occurred as a result of having cooled within the DSC instrument. This is additionally confirmed by the results of the second heating (black).
The modifications formed in the DSC instrument during cooling start to melt already at just over 10°C, which is shown by the endothermal heat of reaction. Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).Melting is already finished at 28 °C, a temperature at which the original chocolate had just barely begun to melt during the first heating. Another important finding is provided by the integral of the melting and crystallization areas. These are proportional to the latent heat values and are therefore a measure for the sample‘s degree of crystallinity. Although the crystalline parts of the sample in its original state led to a melting enthalpy of 49.5 J/g (first heating, red curve), a melting enthalpy of only 30.0 J/g was detected (black curve).
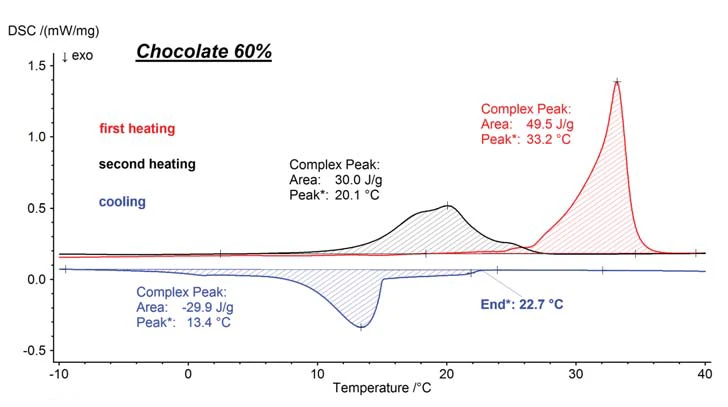
This corresponds to the degree of crystallinity which was obtained during the cooling curve (compare cooling curve, blue). This means not only that – during cooling in the DSC at a linear rate of 5 K/min – different low-melting modifications had occurred than had been the case with the original chocolate production circumstances, but also that the degree of crystallinity had lessened noticeably. This in turn confirms that – as stated above – a special temperature treatment is required for the targeted generation of a large proportion of the high-melting ß-modification.
Variation of the Degree of Crystallization of Chocolate by Means of Tempering
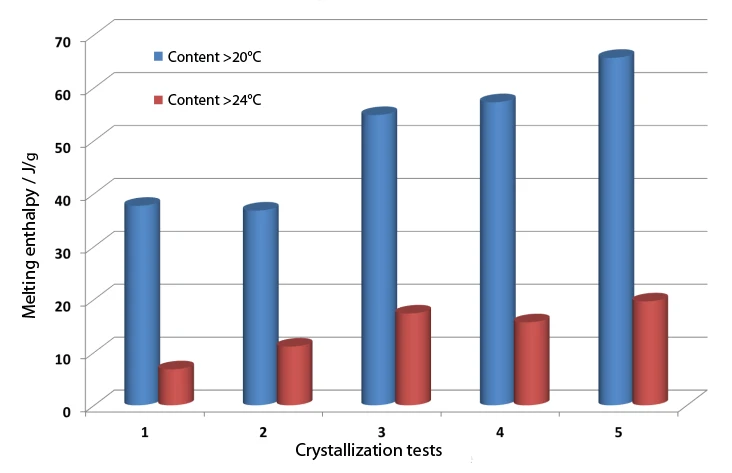
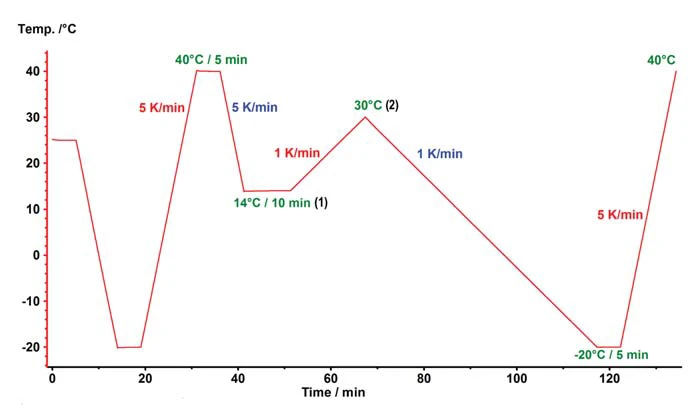
In industrial production of chocolate, the liquid chocolate mass is subjected to a mechanical and thermal treatment to specifi cally obtain the desired high-melting ß-modifi cation and to suppress crystallization of the cocoa butter. Simulation of such a treatment can partially be achieved in the DSC instrument, but understandably without the mechanical component. Figure 5 shows the change in the melting peak area portions above 20°C and above 24°C for a series of tempering tests. CristalizaciónCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.Crystallization test 1 describes the results when a linear cooling rate of 5 K/min is used. Tests 2 through 5 vary the aging temperature (1) and the temperature at which the crystallization nuclei of the undesired PolymorphismPolymorphism is the ability of a solid material to form different crystalline structures (synonyms: forms, modifications).polymorphism are again molten (2). CristalizaciónCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.Crystallization test 5 shows a clear increase in crystallinity as compared to the linear cooling. This was achieved by tempering the sample for 10 minutes at 14° and subsequently heating it to 30°C. The corresponding temperature program is presented in figure 6.
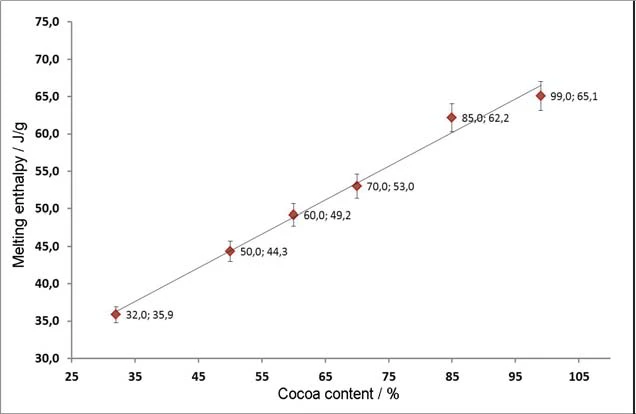
Relationship between Cocoa Content and Melting Enthalpy
Upon investigating chocolates with various levels of cocoa content, it can be seen that the relationship between them is largely linear. As the cocoa content increases, the amount of crystalline cocoa butter also increases and therefore so does the amount of energy necessary for melting. The melting enthalpy can be directly determined from the peak area of the fi rst heating. Applying the nominal cocoa content and the detected melting enthalpy yields a linear relationship, which is shown in fi gure 7. The values listed are the average values for fi ve measurements each. The error bars depicted do not represent the actual measurement errors, but only illustrate that this linear relationship applies with a correlation of + 3%.
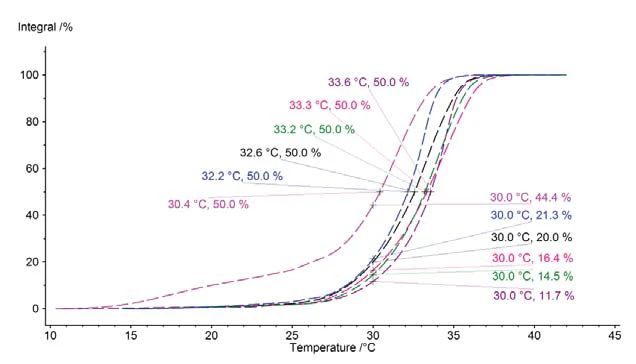
Since the melting peak area aids not only in quantifying the melting behavior of different chocolate samples, but also – by using the position and form of the peak – in determining the temperature range and melting process, it is possible to specify, for each sample individually, how much of the fat (cocoa butter) contained is still solid at the corresponding temperature and how much is already liquid. This information is also known as the Solid Fat Index (SFI). It is easy to arrive at such an assertion if the peak area is scaled to 100% and the course is depicted as a surface integral. Such an application is shown for all of the investigated chocolate samples in figure 8. First of all, it can clearly be seen at which temperature exactly half of the corresponding fat content is still solid; and secondly, it can be easily deducted what portion of the contained fat is already molten at a given temperature (here 30°C) .
Literature contains many examples highlighting the information offered by DSC measurement results besides the ones shown here for the investigation of the melting and crystallization behavior of chocolate. Cammenga et al. describe the use of Differential Scanning Calorimetry for sweets in general. Sugar and sugar substitutes usually constitute the bulk of such products by mass, and measurable properties such as the Temperatura de Transición VítreaThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition temperature, crystallinity, Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature and phase transformation enthalpies – to name just a few – have a major influence on the physiochemical and technological properties as well as on the storage stability [1].
In a whole series of works, Ziegleder et al. decribe the longterm stability [2] and fat bloom formation in chocolate [3].
Chapman et al. [4] and Merken et al. [5] focused on PolymorphismPolymorphism is the ability of a solid material to form different crystalline structures (synonyms: forms, modifications).polymorphism and the processability of chocolate in their work, while Tscheuschner et al. [6] and Ziegleder et al. [7] carried out numerous investigations regarding the cooling conditions and crystallization of chocolate and chocolate mass.
Summary
Cocoa butter crystallizes in six different structures (PolymorphismPolymorphism is the ability of a solid material to form different crystalline structures (synonyms: forms, modifications).polymorphism), of which one – the so-called ß-modification – is preferred for chocolate production. To achieve this result, a special temperature treatment procedure called “tempering“ is necessary. With the help of DSC (Differential Scanning Calorimetry), not only can one determine the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature of cocoa butter which yield information about the modifi cations occurring during production, but one can determine the transformation enthalpy (melting enthalpy) which allows for quantifi cation of the crystalline portions of cocoa butter as well. By investigating various chocolates with a cocoa content of between 32% and 99%, it was possible to confi rm that there is a largely linear relationship between the specifi ed cocoa content and the melting enthalpy as determined by means of DSC. Moreover, it was shown that it is also possible to investigate the infl uence of tempering on the quantity and differentiation of the individual crystalline modifi cations of cocoa butter. The cooling rate in connection with isothermal phases and any subsequent short-term reheating of the chocolate mass all have an infl uence upon the resulting degree of crystallinity. It is thus possible to re-create the tempering of chocolate mass occurring in production within a DSC analysis by means of varying the temperature control. In addition to the fl exible temperature control of the measurement programs in a DSC analysis, the information contained in DSC results yields a number of further safeguarding possibilities for chocolate production in areas such as incoming goods inspection, production control, and quality control.