
17.12.2020 by Dr. Natalie Rudolph, Dr. Stefan Schmölzer
How to Study the Isothermal Crystallization Behavior of SLS Powder Using DSC
In a previous article, the process window in the Selective Laser SinteringSintering is a production process for forming a mechanically strong body out of a ceramic or metallic powder. Sintering process with polyamide 12 powder was determined with dynamic measurements. In this article, we explain how isothermal measurements can be used for more advanced studies.
It was shown that the onset of melting and crystallization are important parameters in the selection of suitable materials as well as in the determination of the process settings. Read the article here! Furthermore, the onset of crystallization is time-dependent and, therefore, isothermal DSC measurements can be used for more advanced studies of SLS materials.
During the SLS process, the molten portions of the part are kept in the molten state to reduce the effects of warpage. However, because the build takes several hours to complete, changes in temperature as well as the prolonged time can lead to crystallization. Read our introduction to the SLS process here!
How to set up the isothermal measurement
The isothermal crystallization behavior of a PA12 powder was studied using a NETZSCH DSC 214 Polyma.
Step 1: The sample was heated from room temperature to above melting at 200°C at 20 K/min. It was kept there for 1 minute to erase the sample history.
Step 2: It was then rapidly cooled to the isothermal temperature step (168, 167, 166, 165, 164, 163, 162°C in Figure 1) using a high cooling rate of 125 K/min to prevent reorganization processes that occur with PA12 at slow cooling rates. Both the ability to achieve a fast cooling rate with regular sample sizes and the ability to precisely control the temperature are features of the DSC 214 Polyma that are extremely valuable for this analysis.
Step 3: Next, the sample was kept at the isothermal temperature for 30 minutes to study the crystallization process.
Step 4: The sample can then be cooled down OR the sample can be heated back up to 200°C at 10 K/min (as was done here) to get the full picture and observe the melting behavior after the isothermal crystallization step. All other measurement conditions are summarized in the following table:
Table 1: Measurement conditions
| Pan | Concavus® Al, unpierced |
| Sample weight | 5 mg |
| Atmosphere | N2 |
| Temperature steps for isothermal measurement at 165°C | 25°C to 200°C (20 K/min), constant for 1 min, 200°C to 165°C (125 K/min), constant for 30 min, 165°C to 200°C (10 K/min), cool down |
Analyzing the crystallization peak temperature
Figure 1 shows the isothermal crystallization behavior at different temperatures from 165°C to 162°C right below the build envelope temperature. The crystallization peak temperature, tmax, is analyzed as the peak of the curve from the start of the measurement. Therefore, the values depicted here were normalized in the Proteus® software for the actual start of the isothermal step.
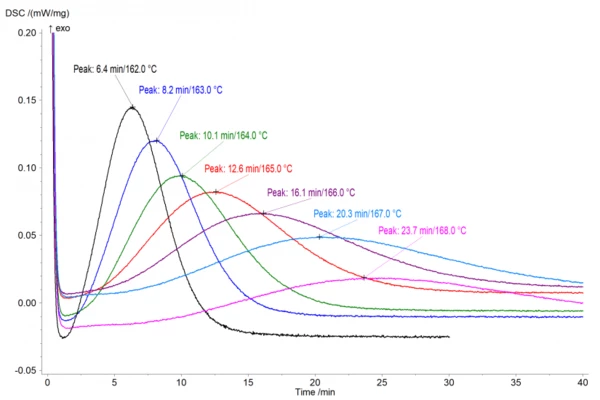
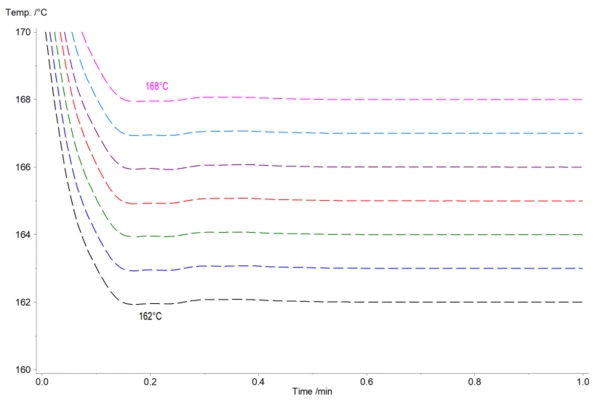
Figure 2 shows the corresponding normalized temperature profile. The isothermal temperatures were reached about 10 minutes after the measurement start. Even at these high cooling rates of 125 K/min, the temperature only overshoots by ± 0.1 K and hits the set temperature in less than 30 s.
What does that mean for my Selective Laser Sintering (SLS) process?
These results highlight that even at a build envelope temperature of 168°C, crystallization starts after about 10 minutes (figure 1) and reaches its peak after 23.7 minutes. While the top layers will be reheated closer to the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature with each additional layer, it becomes obvious that lower layers will eventually stay at 168°C or could even cool further. Thus, given the long build durations of typically several hours, crystallization will occur and has to be taken into account.
To further understand the crystallization rate as a function of time and temperature as well as to model the process – for example, to determine warpage or residual StressStress is defined as a level of force applied on a sample with a well-defined cross section. (Stress = force/area). Samples having a circular or rectangular cross section can be compressed or stretched. Elastic materials like rubber can be stretched up to 5 to 10 times their original length.stress build-up – the crystallization kinetics can be studied. How to set up and interpret these analyses will be shown in future articles.

FREE E-Book
Thermal Analysis and Rheology in Polymer Additive Manufacturing
Discover the secrets behind AM's game-changing capabilities! Our newly released ebook delves deep into the heart of AM, unveiling the power of reliable material characterization techniques, specifically thermal analysis and rheology.