Introduction
Straw is a generic term for threshed, dried grain stalks and the leaves of plants used to produce oils and fibers. In addition to its use in agriculture, straw also has the potential to become important as a CO2-neutral energy carrier in the future. It is an excellent form of biomass because it is a byproduct of arable farming. Unlike other biofuels, no special measures or additional land is required to grow it. The flyash from the burning process can furthermore be used as soil fertilizers for local farms.
Thermogravimetric analysis (TGA) or simultaneous thermal analysis (STA) which refers to simultaneous TGA and differential scanning calorimetry (DSC) are particularly suited for the investigation of PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis or combustion processes. Information about the thermal stability of mostly solid fuels in term of reaction temperatures as well as combustion kinetics can quickly be obtained. Furthermore, the mass loss during PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis or combustion and the ash content can be quantified.
The measurement described herein examines the Réaction de DécompositionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition behavior of straw [1]. The gases evolved during decomposition are identified by means of FT-IR spectroscopy using the fully integrated STA-FT-IR coupling system NETZSCH Perseus STA 449 (see figure 1).

Measurement Results
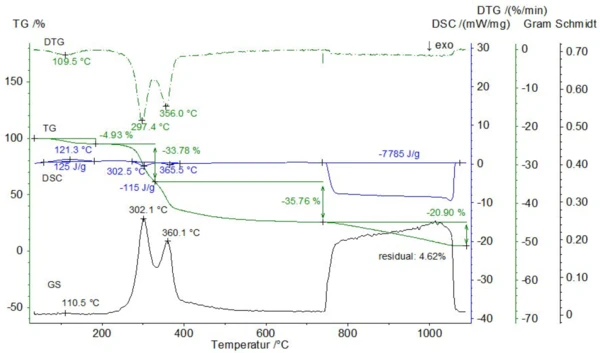
A powdered straw sample of unkown origin with an initial mass of 28.64 mg was measured in a Pt crucible with pierced lid at a heating rate of 20 K/min. The gas atmosphere was changed from pure nitrogen to air at 740°C (the gas flow rates were 70 ml/min). Below 740°C, three mass-loss steps of 4.9%, 33.8% and 35.8% occurred which were accompanied by one endothermic and two overlapping exothermic effects with enthalpies of 125 J/g and -115 J/g (see figure 2). During these mass-loss steps, the Gram-Schmidt signal, reflecting the sum of the entire FT-IR absorbances for all wavenumbers, showed maxima at 111°C, 302°C and 360°C which correlate well with the DTG curve. Another mass-loss step of 20.9% as well as an exothermic effect with a total enthalpy of -7.79 kJ/g occurred after switching to air at 740°C. These effects are due to the combustion of the so called pyrolytic soot, leaving a residual mass of 4.6%, which reflects the ash content.
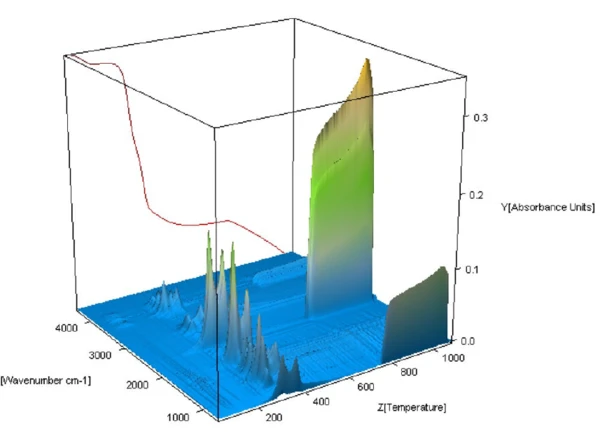
The 3-D view of the FT-IR spectra of the evolved gases collected throughout the decomposition of the straw is shown in figure 3. Of particular interest are the spectra below 740°C where PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis of the sample occurred. The strong FT-IR absorbance at higher temperatures is due to the release of CO2 as a result of combustion.
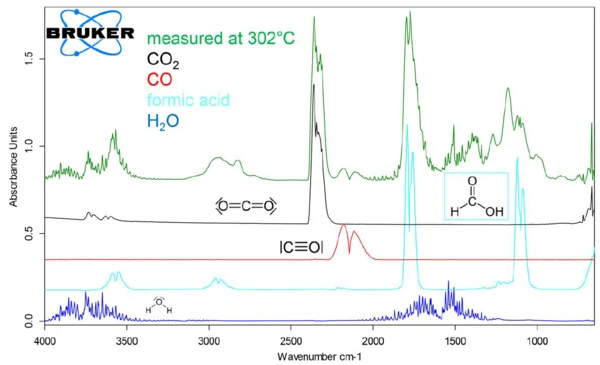
The evolved gas species were identified by comparing individual, extracted 2-D spectra at specific temperatures with library spectra. For example, figure 4 shows that the spectrum of the gases evolved at 302°C is consistent with a mixture containing CO2, CO, H2O, and formic acid (HCOOH). The evolution of individual gas species over the course of the sample decomposition can be traced by integrating a characteristic FT-IR absorbance range for the molecules and overlaying the curve of the integration values as a function of temperature with the TGA and DTG curves from the analysis. The range between 2200 and 2450 cm-1 was integrated for CO2, between 1950 and 2150 cm-1 for CO, between 1300 and 1600 cm-1 for H2O and between 1000-1150 cm-1 for HCOOH.
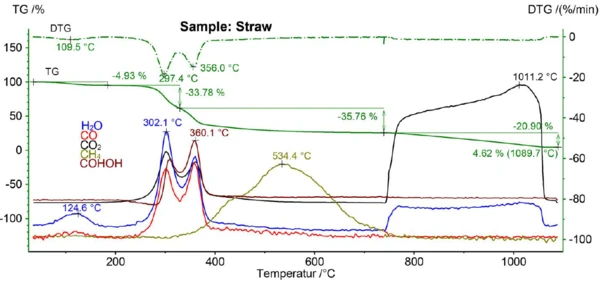
As can be seen from figure 5, H2O was released during the 1st mass-loss step (evaporation of moisture) and during the 2nd and 3rd mass-loss step (PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis), during which CO, CO2 and HCOOH were also evolved. CH4 was evolved over a broad temperature range with a maximum at 534°C and CO2 was detected again above 740°C as a result of the combustion of the sample in air.
Conclusion
Use of the very compact STA-FT-IR coupling system NETZSCH Perseus STA 449 for characterizing the PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis and combustion of straw [1] was demonstrated. Good correlation between the detected mass-loss steps and gas evolution was observed demonstrating the advantage of a direct coupling interface. Identification of the gases evolved by means of a database search allows for detailed interpretation of the chemistry involved in the mass-loss steps associated with PyrolysisPyrolysis is the thermal decomposition of organic compounds in an inert atmosphere.pyrolysis, in particular.