Customer SUCCESS STORY
How to Determine the Thermophysical Properties of Energy Storage Materials
A field report by Dr. Daniel Lager, Research Engineer for Sustainable Thermal Energy Systems at the Center for Energy, AIT Austrian Institute of Technology
The AIT Austrian Institute of Technology (https://www.ait.ac.at/) is Austria’s largest non-university research institution. With its seven Centers, AIT regards itself as a highly specialized research and development partner for industry, and concerns itself with the key infrastructure topics of the future.

„NETZSCH has established itself as a reliable partner. The quality of the instruments and their longevity as well as the usability of the measurement software Proteus® across all measured variables comprise important aspects of the picture. Above all, the good service as well as the good dialogue with the development and application laboratory at NETZSCH have already solved many tricky situations.“
About the Thermophysics laboratory at AIT
The thermophysics laboratory as an accredited testing laboratory (EN ISO/IEC 17025) in the Center for Energy offers measurements of thermal characteristics of materials, processes and products as well as determinations of thermophysical properties and transition parameters with its high-quality and specific laboratory infrastructure and many years of experience. The thermophysical properties analyzed include Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity λ(T), Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity a(T), Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity cp(T), thermal expansion ΔL(T)/L0, coefficient of thermal expansion Coefficient of Linear Thermal Expansion (CLTE/CTE) The coefficient of linear thermal expansion (CLTE) describes the length change of a material as a function of the temperature.CTE α(T), and DensityThe mass density is defined as the ratio between mass and volume. density ρ(T) in a temperature range from -180°C to 1600°C. In addition to the thermophysical properties, simultaneous thermal analysis with infrared and mass spectrometry is used to determine characteristic temperatures, enthalpy differences and mass changes as well as to identify evolved gases.

NETZSCH has established itself as a reliable partner as an equipment manufacturer. The quality of the instruments and their longevity as well as the usability of the measurement software Proteus® across all measured variables comprise important aspects of the picture. Above all, however, the good service as well as the good dialogue with the development and application laboratory at NETZSCH have already solved many a tricky situation.
The oldest instrument currently in use at AIT is the Laser-Flash LFA 427, which has been in operation for more than 20 years:


Phase Change Materials (PCMs) for Thermal Energy Storage Applications
Sensible thermal energy storage (STES) is currently the most common way to store heat by using the heat capacity of the utilized storage material that results from a prevailing temperature difference (e.g., hot water tank). Recent technology includes latent thermal energy storage (LTES), which uses the heat of a phase change of a material. The main difference between using PCMs versus STES materials in a heat storage application is that, in the former, the stored heat lies within a narrow temperature range and the Phase TransitionsThe term phase transition (or phase change) is most commonly used to describe transitions between the solid, liquid and gaseous states.phase transition temperature is constant. This characteristic is used for specific applications, e.g., in building applications. Challenges in the measurement procedure are accurate measurement of the phase change or transition temperature, Tt, the actual Phase TransitionsThe term phase transition (or phase change) is most commonly used to describe transitions between the solid, liquid and gaseous states.phase transition enthalpies, Δht, and the Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity, cp(T), of the different phases.
The investigated PCM was a commercially available paraffin wax with a Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature range from 69°C to 71°C, an enthalpy difference of Δh = 260 kJ kg-1 from 62°C to 77°C, and a Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity of cp = 2 kJ kg-1 K-1according to the manufacturer specification.
The following DSC experiments were conducted with a NETZSCH 204 F1 DSC equipped with a type-E DSC sensor. Aluminum crucibles with a volume of 25 μl were filled with the PCM and cold-welded with lids. The solid organic samples were cut to have one flat side, so as to ensure good contact between the sample and the crucible bottom. The DSC experiments were conducted at two different heating rates, with β = 0.25 K min-1 and β = 10 K min-1, andwith a mass flow controlled nitrogen gas atmosphere.
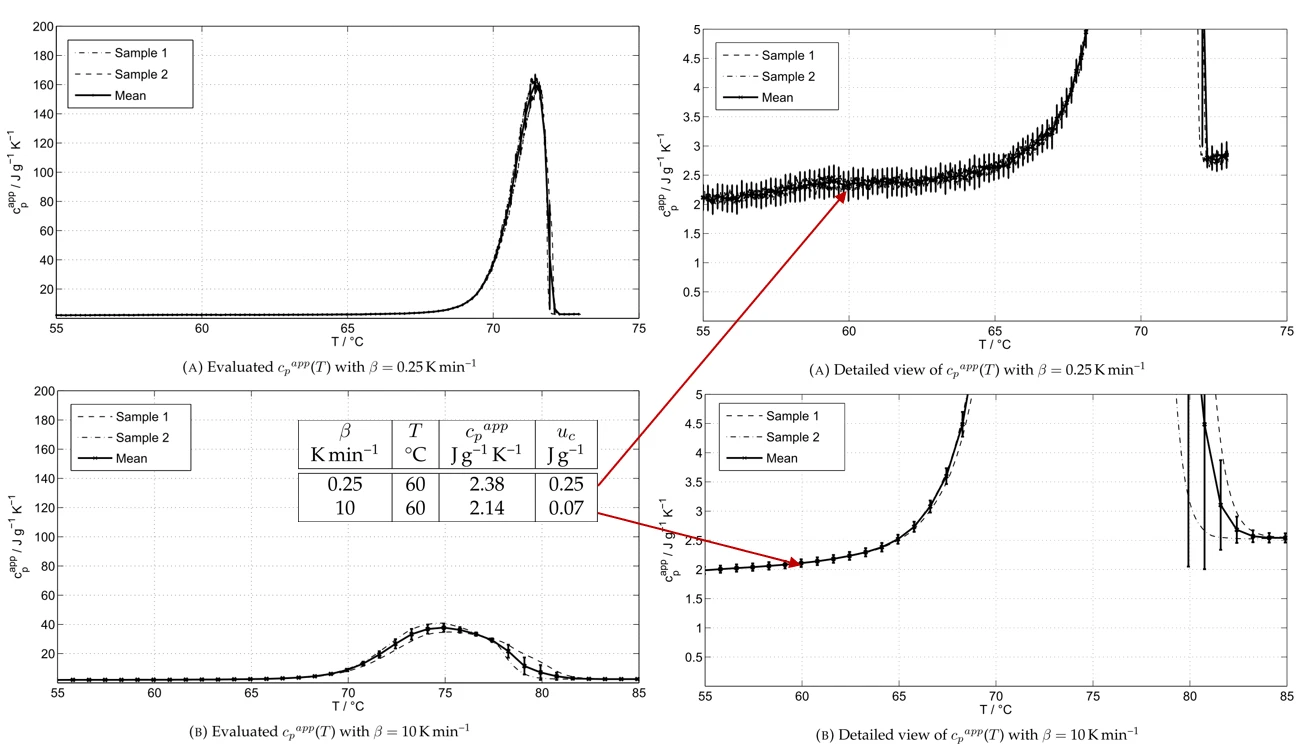
Figure 3 (a): Apparent cp(T) results from DSC measurements with a paraffin wax at β = 0.25 K min-1 and β = 10 K min-1
Figure 3 depicts the results for the DSC measurements on the organic PCM at two different heating rates. The results for the low heating rate with β = 0.25 K min-1 led to a sharp peak but also to high uncertainties in the solid or liquid phase regarding the actual Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity, cp(T). The faster heating rate with β = 10 K min-1 indicates a smeared representation of the melting range but much more accurate results for the actual Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity cp(T) in the solid or liquid phase.
From these results, we concluded that an evaluation of the characteristic temperatures and the transformation enthalpies requires multiple DSC measurements at different heating rates to achieve meaningful results regarding the Phase TransitionsThe term phase transition (or phase change) is most commonly used to describe transitions between the solid, liquid and gaseous states.phase transition temperature and enthalpy and also regarding the specific heat capacity, while excluding thermal transport processes within the sample.
Effective Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.Thermal Conductivity and Specific Heat Capacity Measurements on Battery Cells
The effective Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity λeff(T)in different directions of battery cells as well as their specific heat capacity cp(T) are of essential importance for understanding the thermal behavior and thermal management of battery packs.
The following experiments focused on use of the NETZSCH Laser Flash LFA 427, the NETZSCH DSC 204 F1 Phoenix® and the NETZSCH Heat Flow Meter HFM 446 to evaluate these properties. The LFA 427 and DSC 204 F1 were used to determine the Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity a(T) in the in-plane direction and the cp(T) of anode, cathode, separator, and pouch materials of a dissected lithium-ion pouch cell. The HFM method was applied to evaluate cp(T) and λeff(T)of a lithium-ion pouch cell perpendicular to the pouch surface at a different State of Charge (SoC).
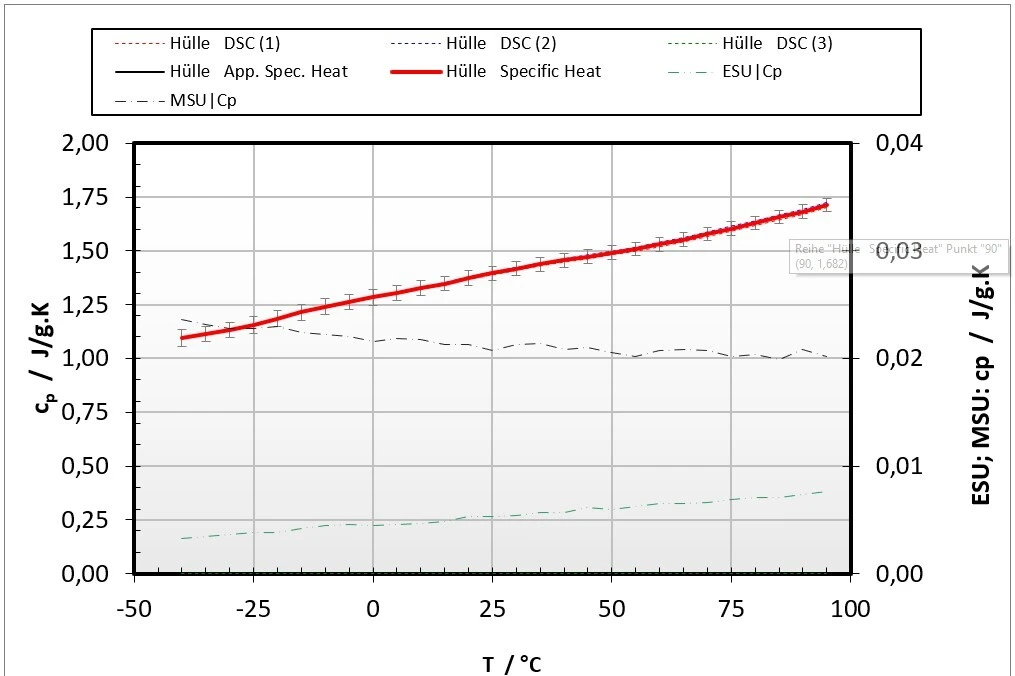
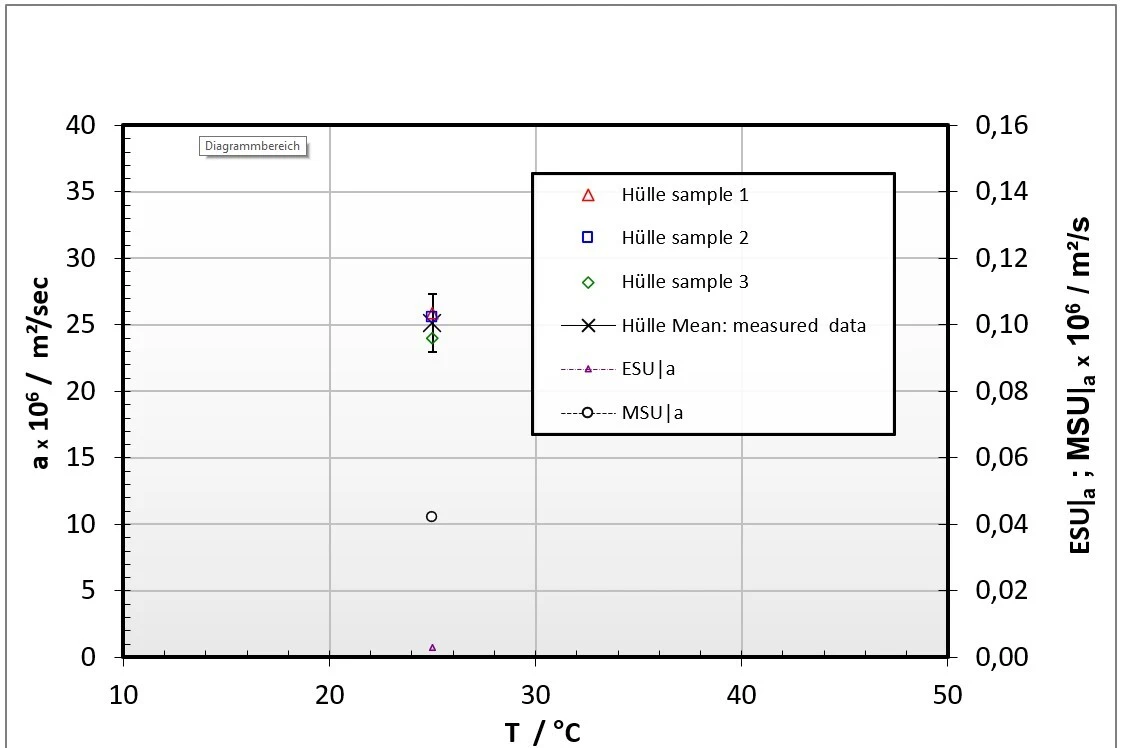
Figure 4: LFA (right) and DSC measurements (left) on the pouch material of a pouch cell
Figure 4 represents the cp(T) and a(T) results for the pouch material of the investigated pouch battery cell. This measurement procedure was conducted with all solid components of the pouch battery cell to evaluate the effective Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity in the in-plane direction based on additional Finite Element calculations.
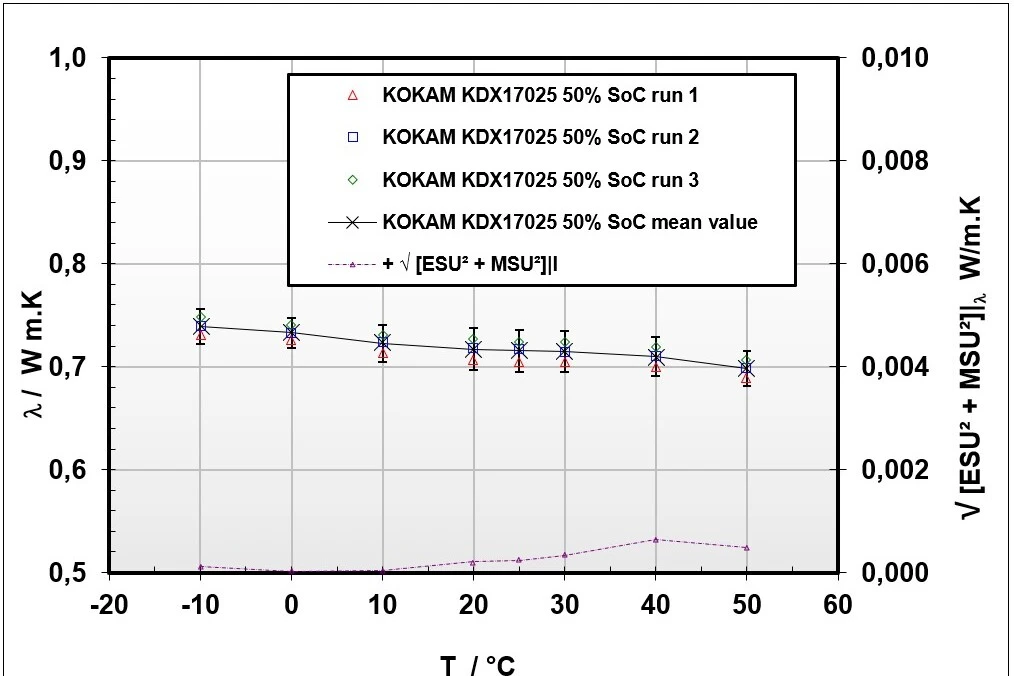
Figure 5: left: Pouch cells stacked in HFM 446; right: Effective Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity based on HFM 446 measurements
Figure 5 depicts the measurement setup for the effective Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity through-plane measurement with the HFM 446 and the pouch cell stack on the left side as well as the received results on the right.
The measurement procedure based on the HFM 446 measurement method with the applied extension set and the pouch cell stack showed good reproducibility for λeff(T)with λeff = 0.715 W m-1 K-1 at T = 25°C and an expanded combined uncertainty of U(k=2) = 0.02 W m-1 K-1. Differences in λeff(T) due to the SoC could not be resolved in the results.
Thedetermined data for cp(T) and a(T) in the in-plane direction of the pouch component were processed in a Finite Element (FE) Model to calculate the in-plane thermal conductivity of the whole pouch cell with λeff = 52.54 W m-1 K-1.
The evaluated results show that HFM is a suitable non-destructive method for analyzing the effective thermal conductivity in the through-plane direction for pouch cells. The effective thermal conductivity in the in-plane direction can be determined by dissecting the cell to its components to determine the in-plane Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity as well as the specific heat capacity and DensityThe mass density is defined as the ratio between mass and volume. density. This data can be processed in an FE Model to evaluate the in-plane effective thermal conductivity.

About the Author:
Dr. Daniel Lager, MSc has been working in the field of Thermophysics and Thermal Analysis since 2007. He is head of the associated accredited laboratory at the AIT Austrian Institute of Technology GmbH as of 2019. In 2017, he received a PhD degree from Vienna University of Technology (TU Wien) for his dissertation on the thermophysical characterization of heat storage materials. He has authored and co-authored numerous publications.
In 2005, he earned a diploma degree in electronics from the University of Applied Sciences Technikum-Wien, followed by a master's degree in biomedical engineering sciences in 2008.
Parallel to his work at AIT, he is an external lecturer at the University of Applied Sciences Burgenland. In the course of his professional career, Daniel Lager has also been able to gain experience as a system physicist for a particle accelerator for ion therapy, as a system engineer for data transmission systems in public safety applications, as a software developer for damage detection systems and as a researcher in the field of the effect of electromagnetic compatibility.
Watch the corresponding webinar!
In this webinar, Dr. Daniel Lager presents state-of-the art measurement methodologies for thermophysical properties of energy storage materials. Focusing on specific heat capacity cp(T), phase transition enthalpy Ht, reaction enthalpy Hr, Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity a(T), thermal conductivity λ(T) and characteristic temperatures T, he performs, compares and evaluates various standardized but also new measurement techniques based on available measurement methods. Watch out now!