
09.05.2022 by Aileen Sammler
How DSC Assists in Characterizing Active Pharmaceutical Ingredients
For the treatment of diseases, the pharmaceutical industry continuously strives for research on new pharmaceutical ingredients (APIs). Dr. Carsten Schauerte from SOLID-CHEM GmbH will show you how DSC can assist in characterizing active pharmaceutical ingredients.
Dr. Carsten Schauerte is co-founder and managing director of SOLID-CHEM GmbH at the Biomedical Center in Bochum, Germany. He graduated with a degree in Chemistry from the University of Essen, received his doctorate in 2004 and worked as a postdoc at the Goethe University in Frankfurt am Main.
At SOLID-CHEM GmbH, founded in 2010, the areas of focus include analysis and development methods for crystallizations, polymorphs, salts and co-crystals, as well as amorphous “screenings” and particle identification and characterization. SOLID-CHEM additionally offers a wide range of analytical methods for cross-linked solid-state analysis.

Today, Dr. Carsten Schauerte gives insight into how DSC supports the characterization of active pharmaceutical ingredients:
For the treatment of diseases, the pharmaceutical industry continuously strives for research on new pharmaceutical ingredients (APIs) featuring specific, purpose-determined, physicochemical properties such as the capability of docking onto receptor proteins and thus triggering desired cell reactions. Once an active pharmaceutical has been found, the challenge is to make it absorbable by the body. The key term here is solubility. In addition, the active ingredient must then be brought into an appropriate dosage form, e.g., tablet, capsule, or solution. The formulation of the drug usually also contains excipients, which serve such functions as exerting a positive influence on solubility or stability. Material characterization plays an important role in this step. From among the wide variety of solid structures (polymorphs, hydrates, solvates, and amorphous materials), those guaranteeing bioavailability and safety of the product must be identified.
Different complementary analytical methods are frequently used for characterization of the respective solid body form. The thermal properties of active ingredients, excipients and formulations can be detected by means of DSC. This includes determination of the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point and general phase transformations, e.g., via EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic signals in the DSC.
Polymorphism of Crystalline Substances – Important for Drug Efficacy
Many crystalline substances are capable of forming polymorphs. Polymorphs are compounds of the same chemical composition, characterized by a different arrangement of molecules within the crystals in the solid state. Different polymorph forms can be generated by setting different parameters during the CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization process from the melt or the solution. They can also be formed by solid-solid phase transformations. These can be favored by humidity or different pressures, but particularly by certain temperatures or temperature gradients. The differences on a molecular level between polymorphs can also cause differences on a macroscopic level. Polymorphs can thus exhibit different physical properties in their various crystalline forms. These include, among others, differing solubility and thus possibly altered bioavailability.
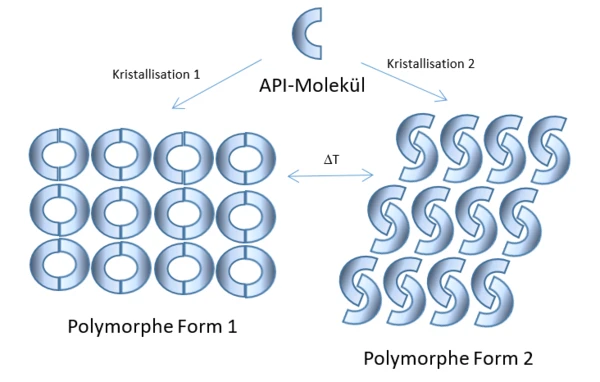
However, finding a stable polymorph with the desired efficacy properties is very time-consuming. Even when a promising substance is found, only one out of multiple thousands of active ingredients “survives” the test phase and manages to become a marketable drug. Such promising active ingredients are therefore also patented by pharmaceutical companies to guarantee exclusive marketability.
Analysis as a Helpful Tool for Troubleshooting in Drug Manufacture
In-depth laboratory studies provide information on the optimum processing parameters for each polymorphic form such as its solubility, preferred CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization solvent, optimized concentrations in mixed solvent systems, CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization conditions, and more. If a drug, however, does not exhibit its desired efficacy in use, it is necessary to clarify at which point of processing or preparation the problems occur. Maybe the active ingredient has changed to another polymorphic form as a result of the production process or an undesirable interaction with excipients, or perhaps the issue is caused by impurity in the product? In such cases, pharmaceutical companies often enlist the assistance of specialized contract laboratories like SOLID-CHEM GmbH in Bochum, Germany. Extensive analysis methods are available in their in-house laboratory, such as X-ray and laser diffraction, VibrationA mechanic process of oscillation is called vibration. Vibration is a mechanical phenomenon whereby oscillations occur about an equilibrium point. In many cases, vibration is undesirable, wasting energy and creating unwanted sound. For example, the vibrational motions of engines, electric motors, or any mechanical device in operation are typically unwanted. Such vibrations could be caused by imbalances in the rotating parts, uneven friction, or the meshing of gear teeth. Careful designs usually minimize unwanted vibrations.vibration and nuclear magnetic resonance spectroscopy, and microscopy, as well as thermal analysis with thermogravimetry and differential scanning calorimetry by means of a NETZSCH DSC 204 F1 Phoenix®.
How Can Thermal Analysis Help?
Thermal analysis comprises a series of methods. One of these is differential scanning calorimetry (DSC), used to test whether Phase TransitionsThe term phase transition (or phase change) is most commonly used to describe transitions between the solid, liquid and gaseous states.phase transitions or chemical reactions occur in a material. To this end, the sample is subjected to a defined temperature program, i.e., the temperature at the sample is increased or decreased at a specific rate or left constant for a certain time. The adsorbed (ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal) or absorbed (endothermal) heat is measured. This allows conclusions to be drawn about chemical and physical processes such as melting, CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization or polymorphic transformations.
The Occurrence and Recognition of Polymorphic Forms with the Example of Paracetamol
Three polymorphs are known for the active ingredient paracetamol, a common pain reliever:
- Stable form I (monoclinic)
- Metastable form II (orthorhombic) and
- Instable form III
The different polymorphic forms can be well distinguished by means of DSC analysis.
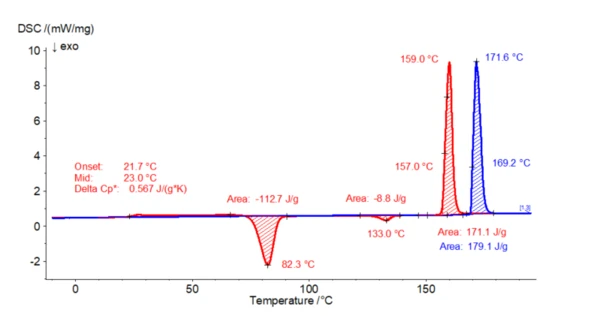
In the following example, 2.4 mg of paracetamol was heated twice from -20°C to 200°C in a nitrogen atmosphere in aluminum crucibles. The cooling segment in between was also carried out at 10 K/min. In the first heating, an endothermal effect with an extrapolated onset temperature of 169°C can be seen. This correlates well with the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point of form I. During the subsequent controlled cooling step (not shown here), no CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization takes place. This means that paracetamol is still amorphous at the beginning of the 2nd heating step. During the 2nd heating, first a Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition (small step in the endothermal direction) occurs as a characteristic of the amorphous state, followed by an ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal effect (with a peak temperature of 82°C) related to a cold- or Post Crystallization (Cold Crystallization)The post crystallization of semi-crystalline plastics occurs primarily at elevated temperatures and increased molecular mobility above the glass transition.post-crystallization process. Parallel XRD studies have shown that form III is formed here. This form III transforms into form II upon further heating (also confirmed by XRD investigations), which finally melts at 157°C (extrapolated onset temperature). The ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal effect at 133°C (peak temperature) is due to the structural transformation into the other polymorphic form. The extrapolated initial temperature of 157°C is characteristic of form II.
We asked Dr. Schauerte a few more questions to complement his article:
NETZSCH: Dr. Schauerte, You collaborate closely with pharmaceutical companies, providing support for problems encountered during the development and processing of pharmaceutical active ingredients. What are the questions most commonly asked by pharmaceutical companies and how can (thermal) analysis methods help solve these problems?
Dr. Carsten Schauerte: In terms of polymorphic systems, the most commonly asked questions are:
- What solid forms exist?
- What are the properties of the respective forms?
Especially for the first question, the answer is not easy and extensive experiments with subsequent cross-linked analysis must be planned and carried out to describe the solid-state landscape of a drug candidate as accurately as possible. This always depends on how much time and energy (and financial resources) are to be invested. Analytical methods are particularly useful here for identifying and characterizing new polymorphic forms. Thermal analyses show the thermal behavior of the different forms (Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transitions, melting andCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization and also outgassing of liquids), but also yield information on potential transformation properties between two or more forms. In addition, DSC can, for example, also be used as a preparative tool for the generation of new forms.
NETZSCH: The high investment expenditures incurred by a pharmaceutical company until a marketable active ingredient is found mean that patent law issues are also part of your scope. Could you briefly explain what this is mainly about and the ways in which (thermal) analytical methods also contribute to solving these problems?
Dr. Carsten Schauerte: Applications for patents on a polymorphic solid form are usually submitted as follow-ups to the expiration of a substance patent and often serve to extend the patent protection for the active substance. Other companies can then challenge this new patent or market an alternative, unprotected form, possibly even having it protected themselves. Thermal analyses also contribute here to the characterization and clear assignment of forms. Moreover, by determining the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point, for example, they may clarify the decisive advantage of a new form over other forms, which can lead to patenting.
NETZSCH: One last question for you, Dr. Schauerte: Differential scanning calorimetry is one of the most frequently used thermal analysis methods. Where do you see the strength of DSC in your applications?
Dr. Carsten Schauerte: As important and valuable as the methods of X-ray diffraction, microscopy and vibrational spectroscopy are, they usually only provide a snapshot while thermal analysis methods present a dynamic picture over a defined temperature range. This is of utmost importance to us since active ingredients are handled not only at one very specific temperature, but at multiple temperatures over the course of time: There are manufacturing and formulation processes as well asstorage and transport routes during which the respective active ingredient is exposed to higher or lower temperatures, and the selected solid-state form must withstand these. To guarantee this, we need to know and describe the thermal behavior of the active ingredient or polymorph as precisely as possible in order to be able to prevent undesirable phase transformations.
NETZSCH: Dr. Schauerte, thank you very much for this exciting insight into your work!