Introduction
Clavulanic acid is a β-lactamase inhibitor drug, which strengthens the effect of an antibiotic against infection. Administered alone, it is only capable of weak antibacterial activity against most organisms, but given in combination with other ß-lactam antibiotics, it prevents antibiotic inactivation by microbial lactamase [1].
It is commonly used as potassium salt, potassium clavulanate, because this substance is more stable and less hygroscopic than clavulanic acid. However, potassium clavulanate is still extremely hygroscopic and susceptible to hydrolysis if stored in a humid environment [3]. This makes it necessary to choose the storage conditions carefully. Additionally, the percentage of water in the components used for pharmaceutical formulations containing potassium clavulanate must be taken into consideration.
In the following, the influence of humidity on the thermal degradation of potassium clavulanate is investigated by means of TGA-FT-IR.
Test Conditions
Three samples of potassium clavulanate were tested: the original substance and two additional samples, which were stored in an open vessel placed above the water in a sealed water container. One sample of the samples from within the water container was tested after one week of storage; the second was tested after two weeks.
All three samples (without treatment, after one week and after two weeks in a humid atmosphere) were prepared in sealed aluminum crucibles.
The TGA measurements were carried out with the TG 209 F1 Libra® under a dynamic nitrogen atmosphere (40 ml/min). A piercing device automatically pierced the crucible lid just prior to the measurement. The gases evolved during heating at 10 K/min to 600°C were directly transferred via the transfer line into the FT-IR spectrometer by Bruker Optics.
Test Results
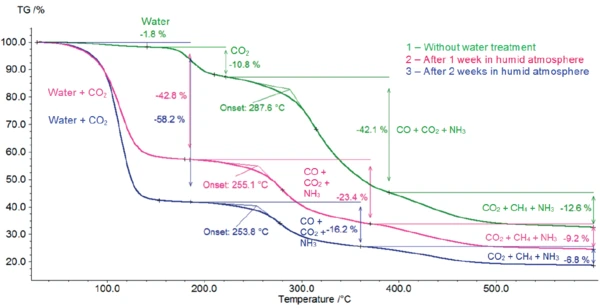
Figure 2 depicts the mass changes of potassium clavulanate with and without water treatment. As soon as heating starts, an initial mass loss occurs.
The sample stored for one week in water exhibits a massloss step of 43%. 58% mass loss occurs in the sample with 2-week storage. For the original sample, this massloss step amounts to 1.8%.
Figures 3, 4 and 5 show a 3-D representation of the FT-IR spectra of the gases released during heating of the three different samples.
During the first mass-loss step of potassium clavulanate without water treatment, only the release of water can be detected (see NETZSCH application note 118/2018 [4]).
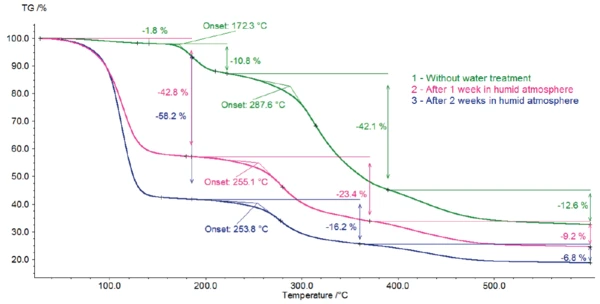
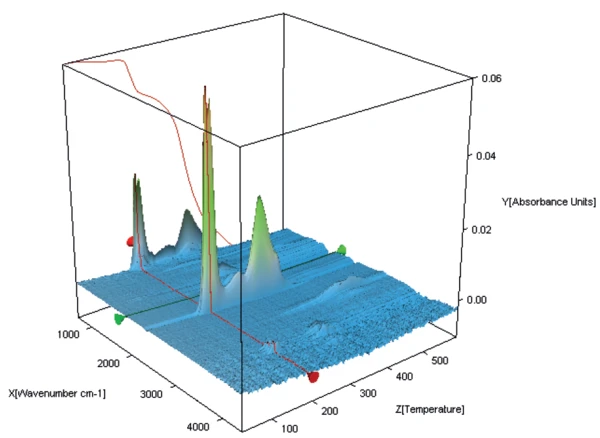
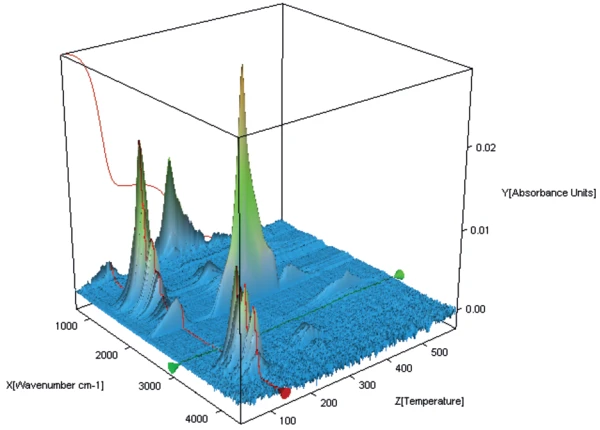
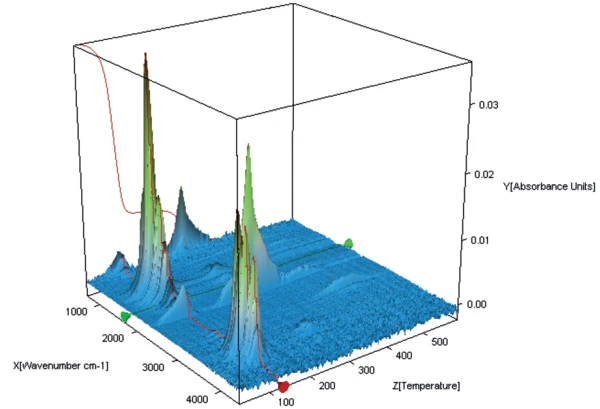
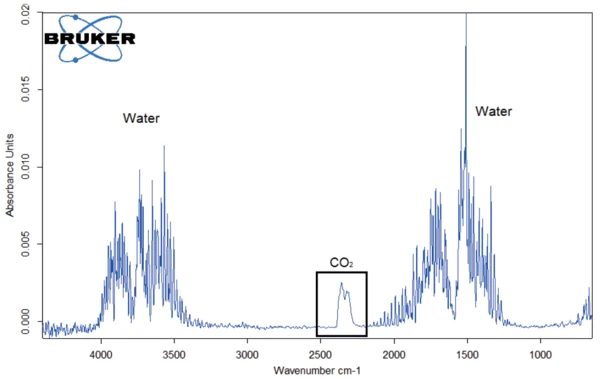
Figure 6 shows the FT-IR spectrum of the gases evolved at 119°C from potassium clavulanate stored for one week in a humid atmosphere. In addition to the typical FT-IR spectrum of water, the bands between 2200 cm-1 and 2400 cm-1 prove the presence of carbon dioxide. The mass-loss step of 43% therefore results from an overlapping release of water and CO2, indicating the start of the clavulanate Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition.
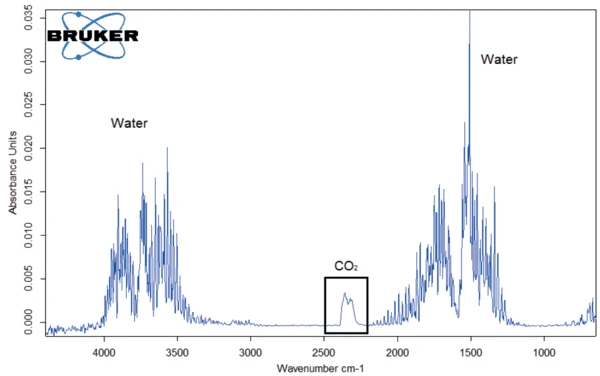
The same conclusion can be drawn from the spectrum at 119°C of the sample stored for two weeks (figure 7).
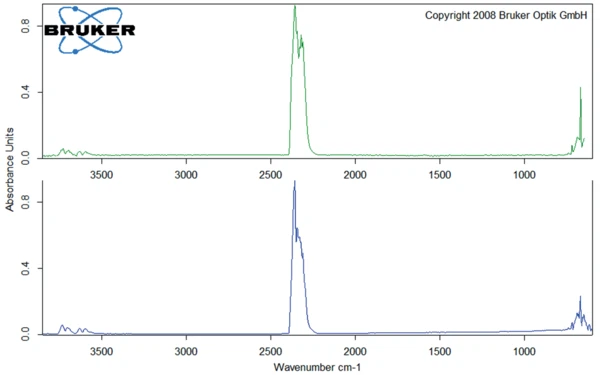
In the sample without water treatment, Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition starts at 172°C (onset temperature of the TGA curve) with the release of solely CO2 (figure 8).
For all three samples, Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition continues with two additional steps, with mass losses of 42% and 13% for the sample without storage, 23% and 9% for the sample after 1-week storage and 16% and 7% for potassium clavulanate stored for 2 weeks.
The first of these steps above 200°C is associated with the release of carbon dioxide and carbon monoxide. The presence of ammonia can also be detected, but only in low concentrations (figure 9). The longer the duration of the water treatment, the lower the temperature at which this mass loss occurs, beginning at 288°C for the sample without storage and at 254°C for potassium clavulanate stored for 2 weeks in a humid atmosphere.
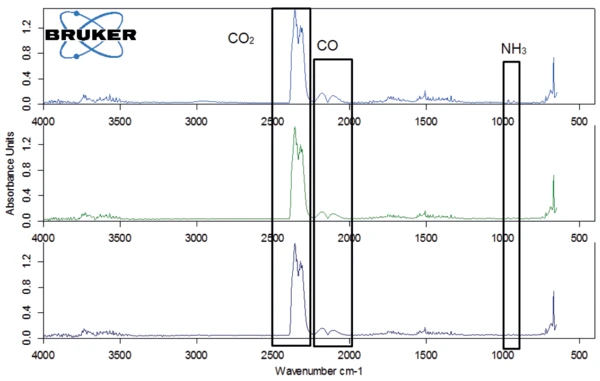
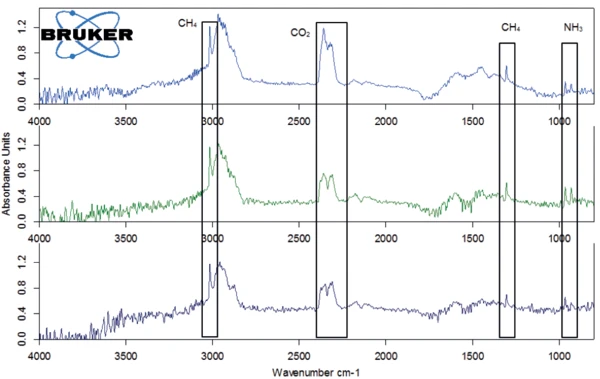
The last mass-loss step, between approx. 380°C and 600°C shows a similar gas composition: In the FT-IR spectrum, methane, carbon dioxide and ammonia occur next to each other at 450°C (figure 10). The results of the TGA-FT-IR measurements on the three samples are recapitulated in figure 11, including the conclusions drawn from the coupling experiment.
Conclusion
Potassium clavulanate exhibits a tendency for hydrolysis [3]. In order to explore the consequences of this property, potassium clavulanate was stored for different periods in a humid atmosphere. By means of TGA, differences in hydrolysis can be recognized. The samples stored in a humid atmosphere exhibit only three massloss steps, whereas the untreated sample exhibits four mass-loss steps.
TGA-FT-IR coupling allows for analysis of the gases evolved during heating of the treated and untreated samples. It clearly illustrates that the first-mass loss step of the samples stored in a humid atmosphere is not due solely to the release of water, but also to that of CO2. This fact already points to the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of the substance. As a result of storage in a humid atmosphere, the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition temperature of potassium clavulanate shifts to lower temperatures. This may be the reason that it is recommended for potassium clavulanate to be stored between +2°C and +8°C [5]. The subsequent mass-loss steps represent similar processes in all three samples, as the same gases are released – irrespective of storage in a humid atmosphere.
The influence of moisture on potassium clavulanate must be taken into account when storing pharmaceuticals under different climatic conditions. Especially in tropical countries with high humidities and temperatures, it should be ensured that the shelf life is not reduced by Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition during storage.