Introduction
Injection molding is the primary process in the polymer industry for producing parts of a defined shape. The molten polymer is injected into a relatively cold mold cavity where it is rapidly cooled. The temperature of the mold directly influences the CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization rate and thus the properties of the final product, so it must be perfectly defined. To this end, the use of a DSC for IsothermalTests at controlled and constant temperature are called isothermal.isothermal CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization tests, where the behavior of a polymer in the mold is simulated, is a real gain in time.
Fast Cooling and Stabilization
For IsothermalTests at controlled and constant temperature are called isothermal.isothermal CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization tests, a DSC must fulfill two requirements. The sample must be cooled very quickly to prevent the start of CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization during cooling. In addition, the temperature must be stabilized at the specified CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization temperature without any under- or overshooting. Particularly a temperature undershot can lead to the premature start ofCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization. Some polymers such as polyolefins crystallize very fast. Only a few seconds at a temperature slightly below the target temperature can unintentionally startCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization.
Thanks to the low thermal mass of its furnace, the P-Module of the DSC 300 Caliris® achieves very fast heating and cooling rates as well as excellent temperature control during subsequent IsothermalTests at controlled and constant temperature are called isothermal.isothermal segments.
In this example, IsothermalTests at controlled and constant temperature are called isothermal.isothermalCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization tests were carried out on a high-DensityThe mass density is defined as the ratio between mass and volume. density polyethylene with the NETZSCH DSC 300 Caliris®. After heating to 230°C, i.e., to a temperature higher than the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature of HDPE (High DensityThe mass density is defined as the ratio between mass and volume. Density Polyethylen), followed by a 5-minute IsothermalTests at controlled and constant temperature are called isothermal.isothermal segment, the samples were cooled down at a high cooling rate to three differentCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization temperatures. Table 1 details the measurement conditions.
Table 1: Conditions for the IsothermalTests at controlled and constant temperature are called isothermal.isothermalCrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released. crystallization tests
| Device | DSC 300 Caliris® with P-Module | ||
| Crucible | Concavus® (aluminum), pierced lid | ||
| Sample mass | 5.55 mg | 5.68 mg | 5.58 mg |
| Temperature range | 230°C to temperature of crystallization | ||
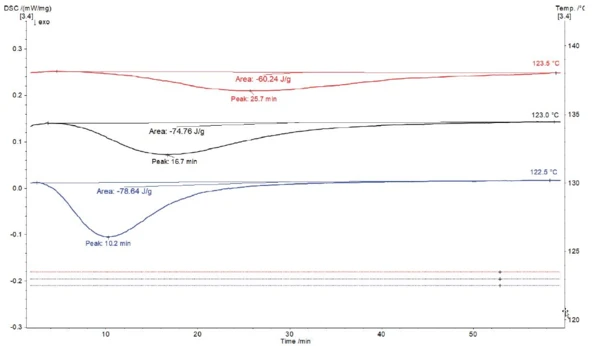
| Temperature of crystallization | 122.5°C | 123.0°C | 123.5°C |
| Nominal cooling rate | 200 K/min | ||
| Atmosphere | Nitrogen (40 ml/min) | ||
Measurement Results and Discussion
The temperature profile of the cooling to 123.0°C demonstrates the excellent stability of the temperature during the IsothermalTests at controlled and constant temperature are called isothermal.isothermal segment after the targeted crystallization temperature was reached (figure 1).

Figure 2 presents the resulting DSC curves for the IsothermalTests at controlled and constant temperature are called isothermal.isothermal segments at 122.5°C, 123.0°C and 123.5°C. Due to the fast stabilization of the temperature at the specified value, the initial effect on the DSC curve caused by the segment change from cooling to IsothermalTests at controlled and constant temperature are called isothermal.isothermal is low enough to allow separation from the thermal effects occurring at its beginning. The ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermal peak detected during the IsothermalTests at controlled and constant temperature are called isothermal.isothermal segment of the three measurements can be attributed to the crystallization of polyethylene. As expected, the crystallization enthalpy (peak area) increases as the temperature of the isothermal segment decreases, indicating a higher degree of Crystallinity / Degree of CrystallinityCrystallinity refers to the degree of structural order of a solid. In a crystal, the arrangement of atoms or molecules is consistent and repetitive. Many materials such as glass ceramics and some polymers can be prepared in such a way as to produce a mixture of crystalline and amorphous regions.crystallinity in the final product. Also, the slope of the peak is steeper with decreasing isothermal temperature, so the peak minimum is reached faster. This signifies a faster crystallization.
From DSC Measurements to the Kinetics of Crystallization: Kinetics Neo
The dependence of the crystallization peak on the temperature allows for the use of DSC curves for a kinetics analysis of the crystallization process. For this, the Kinetics Neo software was used. It can assign each individual step different reaction types with kinetic parameters of their own, such as activation energy, order of reaction, and pre-exponential factor.
The rate of chemical reaction for each crystallization step, j, can be written as the product of two functions, where the first function, fj(ej,pj,), depends on the concentrations of the reactant (ej) and product (pj). The second function, Kj(T), depends on temperature [1].

Here, a one-step reaction was selected for the crystallization kinetics. CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.Crystallization model by Sbirrazzuoli [2] uses the Nakamura dependence K(T) und Sestak- Berggren dependence on concentrations f(e,p):
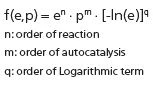
Use of this model requires knowledge of the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition and Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature of the sample, even if the software will be optimizing the value of the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature. The kinetics evaluation will then be valid across the entire temperature range between those two temperatures.
Additionally, the function K(T) includes the parameters U and KG which are optimized by the Kinetics Neo software.
Figure 3 depicts the measurement curves as well as the curves calculated in Kinetics Neo using the kinetics model described above. Table 2 summarizes the parameters of the kinetics. The results show the good accordance between the measured and the calculated results. The coefficient of correlation amounts to 0.996.
Table 2: Parameters of the crystallization kinetics
| Reaction type | Sbirrazzuoli crystallization |
| Nakamura KG | 24.384 |
| Log(PreExp) [Log(1/2)] | 2.072 |
| Order of reaction, n | 1.286 |
| Order of autocatalysis, m | 0.695 |
| Order of logarithmic term, q | 0 |
| Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).Melting temperature [°C] | 130 |
| Glass transition temperature [°C] | -130 |
| U* [kJ/mol] | 6.30 |

Based on the results, Kinetics Neo is capable of simulating the reaction for user-specified temperature programs. For example, figure 4 displays the DSC curves obtained for crystallization temperatures between 80°C and 115°C. As expected, the lower the temperature, the faster the reaction. If the material is injected into a small mold at a temperature of 80°C, it will crystallize in a few seconds. If the mold is at 115°C, the polymer will require one minute for complete crystallization.

DSC Tests Accompanying Production for Saving Time and Money
IsothermalTests at controlled and constant temperature are called isothermal.Isothermal crystallization tests can be carried out with the NETZSCH DSC 300 Caliris®® on polyethylene – a polyolefin known for its fast crystallization. DSC tests are easy to carry out and require only a small sample mass. In particular, isothermal crystallization measurements help determine appropriate processing conditions such as mold temperature and cooling time so that the resulting parts have all the properties required.