Introduction
Freeze-drying (lyophilization) is a technique widely used in pharmaceutical technologies to transform thermolabile substances such as proteins or liposomes – without thermal treatment – into usable and storable forms. The objective of lyophilization is the gentle removal of water from solutions to obtain a stable powder of defined residual moisture and porosity.
A product’s composition has a decisive influence on the process parameters and thus also has an effect on the type, quality and stability of the resulting lyophilisate. Dynamic scanning calorimetry (DSC) provides important information for the selection of appropriate conditions.
The solutions to be lyophilized are usually complex multi-component systems consisting of active ingredients, additives and water. The auxiliaries include toning salts (for adjustment of isotonicity), buffer substances, cryoprotectors (for protection against damage during freezing) and builders that give structure to the freeze-dried product. Sugars such as sucrose or trehalose have proven to be very effective in stabilizing proteins [5]. The following considerations are based on sucrose as a model substance. The solutions mentioned were produced from commercially available sucrose of pharmaceutical quality (Caesar & Loretz, Hilden) and double-distilled water.
The lyophilization process can generally be divided into 3 consecutive steps:
Deep-Freezing
Sugar solutions tend to oversaturate. Upon cooling, ice and an increasingly viscous sucrose solution are formed. The increasing viscosity complicates diffusion processes, which would be necessary for CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization. As a result, the system does not crystallize but solidifies as an undercooled liquid without complete phase separation (glass). The Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition temperature of the maximally concentrated solution is designated Tg’ and is substance-specific [3].
During cooling, supercooling can often be observed. Pharmaceutical solutions for parenteral application (administration avoiding the gastrointestinal tract), which must be particle-free, represent an extreme case. They have virtually no heterogeneous impurities that could act as CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization nuclei. Therefore, crystal nucleation in such solutions is often only likely as the temperature approaches -40°C.
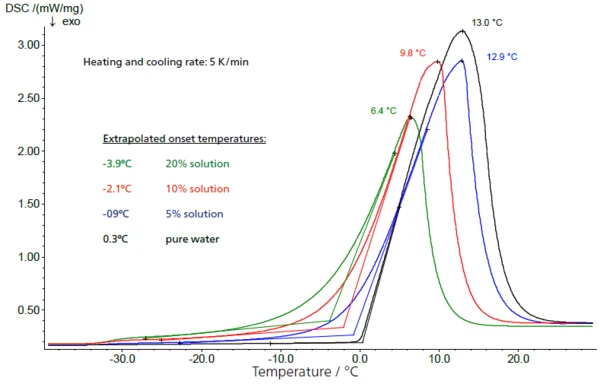
The freezing behavior of a 10% sucrose solution is shown in figure 2. The sample was cooled with the NETZSCH DSC 204 F1 (see figure 1) in a closed aluminum crucible at a controlled cooling rate of 5 K/min. The supercooled solution solidifies extremely quickly at -20°C (extrapolated onset temperature).

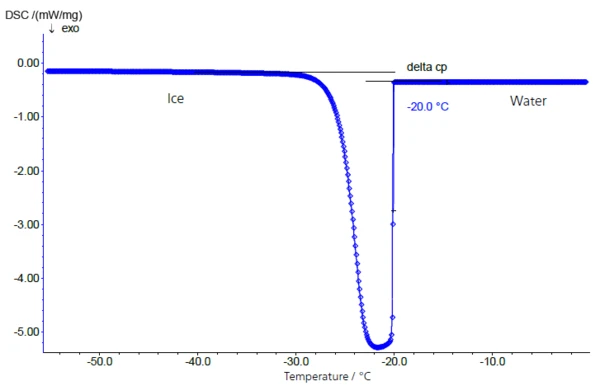
Rough spots on the inside of the crucible or traces of contamination caused by preparation can serve as seed crystals. For this reason, solidification temperatures determined this way can generally not be correlated with the concentration of the sugar solutions used.
During the water-to-ice transition, a change in specific heat from 4.18 J/g·K (water) to 2.1 J/g·K (ice, just below the freezing point) occurs, which is primarily responsible for the clear baseline shift before and after the solidification/melting peak (fig. 2: water-to-ice transition – and figure 3: ice-to-water transition).
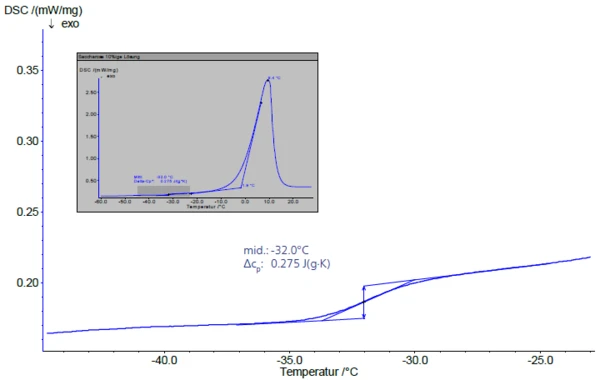
In the subsequent heating at a heating rate of 5 K/min (figure 3), the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition of the maximally concentrated solution appears at -32°C (midpoint). This value is in good agreement with literature data assuming -32°C and -33°C [2], [4].
The Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition is followed by an endothermal peak during heating (inset in figure 3), the extrapolated onset temperature of which, Tm´, describes the start of melting of the ice. According to Roos [1], the maximum “freeze concentration” can only be observed at freezing temperatures between Tg´ and Tm´.
The area below the melting peak corresponds to the free water portion. The reference point here is the heat of fusion of the ice of 333.7 J/g.
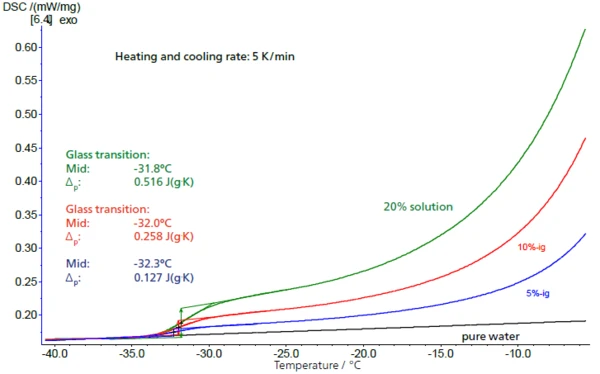
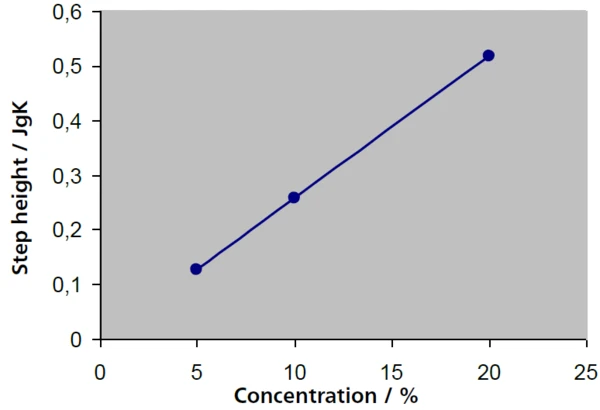
In low-concentration solutions, the sucrose proportion can be determined from the height of the respective Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition. In figure 4, the step heights (ΔSpecific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.cp values) for solutions of 5%, 10% and 20% – with results of 0.127 J/g·K, 0.258 J/g·K and 0.516 J/g·K – are in very good agreement with a scaling of the concentration by a factor of 2, while the glass transition temperatures remain largely constant. There is a linear relationship between the step height and the concentration (fig. 5).
Additionally, as the concentration of the sucrose solutions increases, the start of the melting of ice (extrapolated onset temperature) is shifted to lower values in figure 6. At higher concentrations, this results in a lower interval between the glass transition of the maximally concentrated solution and the start of melting of the free water.
Some amorphous substances again crystallize upon heating above the glass temperature. This effect, called devitrification or cold CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization, can be used to change the porosity and residual moisture of the lyophilisate [2] by tempering of the material above the recrystallization temperature (extrapolated onset). Due to recrystallization, a phase separation occurs and the “unfrozen” water released turns to ice. As shown in figure 3, however, no post-CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization occurs in the case of sucrose.
Primary Drying
In this step, the frozen ice is removed in a vacuum by sublimation (transition from the solid to the gaseous aggregate state).
During this process – in which heat is supplied from the outside – the temperature in the product should not rise above the temperature of the glass transition since this leads to softening of the framework structure and collapse of the system [5]. The destruction of the framework structure during the drying phase is called collapse.
Although collapse temperatures are reported that are an average of 1 to 5 K higher than the corresponding glass transition temperatures [6], the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transitions of the maximally concentrated solutions, Tg´, which can be determined by means of DSC, are good reference points for their position.
Secondary Drying
In this step, the product is dried to the desired final moisture level by desorbing the water contained in the matrix via a slow increase in temperature.
In amorphous lyophilisates, the water must diffuse from the glassy phase to the surface. This rather slow process is the reason that the post-drying step is often the one which determines the speed of freeze drying for amorphous lyophilisates [2].
Due to the softener effect of water, the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition temperature of the amorphous phase is directly related to the entrapped water content. As dewatering progresses, the Tg (the glass transition of sucrose as a solid) increases; its position can also be determined quickly and precisely by means of DSC.
Conclusion
Essential characteristics for designing the primary drying process are the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition temperature of the maximally concentrated solution (Tg´) and the collapse temperature at which the material softens so that it can no longer support its own structure and begins to flow. Using DSC (sometimes as TM-DSC*), the Tg´ can easily be determined.
The collapse temperature is slightly higher than the Tg´; the exact interval between the Tg´ and the collapse temperature is formulation-dependent.