Introduction
The laser flash analysis (LFA) method allows for the fast and easy measurement of the Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity of a variety of materials – from metals to polymers to ceramics. From the Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity and specific heat, the Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity of the material can be calculated. In the LFA measurement, the front surface of a sample is heated by a flash lamp or laser pulse and the temperature increase on the rear surface is recorded by means of an infrared detector.
In order to obtain a good detector signal, the sample must fulfill some important criteria:
- The sample must not be translucent in the visible and near-IR wave length range
- The sample must not reflect light
- The sample must feature good emission and absorption capability
Not all materials automatically satisfy these criteria. Many polymers and glasses are translucent in the visible and near-IR wave length range. Metals, on the other hand, are highly reflective. Additionally, most materials feature low emission/absorption capability, which reduces the signalto- noise ratio. In these cases, in order to obtain good signals, the samples are either coated with graphite or sputtered with gold. This application note describes how the coating is applied to the different samples and the influences the coating can have on the measurement result.
When is a Coating Required?
In general, all samples should be coated. A coating improves a sample‘s emission/absorption properties, optimizing the signal-to-noise ratio. Figure 1 shows the signal of a sample with and without coating. The signal-to-noise ratio and curve resolution are significantly worse for the sample without the coating.
Only few samples that are non-reflective and opaque (e.g., carbon-containing samples) need not to be coated. Shown in figure 2 are the signals of a graphite-containing polymer sample with and without graphite coating. Since this sample is not translucent and does not reflect, both signals are almost identical and a coating is not necessarily required for measurement of the Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity.
A coating is absolutely necessary if the Specific Heat Capacity (cp)Heat capacity is a material-specific physical quantity, determined by the amount of heat supplied to specimen, divided by the resulting temperature increase. The specific heat capacity is related to a unit mass of the specimen.specific heat capacity of the sample will be measured against a reference using the LFA. Sample and reference require the same emission/ absorption capability. This can be achieved by means of a graphite layer.
Which Coating is to Apply and When?
Graphite is the standard coating. It is applied as a graphite spray and dries on the sample to form a graphite layer.
For very thin, transparent samples, e.g., PE films, graphite layer can be too thick compared to the sample in order to eliminate light transmission. It is better in this case to sputter a gold layer onto the sample to make it opaque. The gold-coated sample should then be dusted with graphite in order to increase its emissivity/absorptivity.
In cases where the carbon can potentially react with the sample, especially at high temperatures (e.g., for steels), a different coating may be necessary. Often simply roughening of the surface, e.g., by sandblasting or abrasive paper, is sufficient.
How Thickly Should the Coating be Applied?
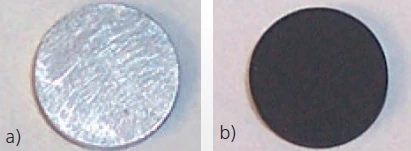
For most samples, an even graphite layer of approx. 5 μm that thoroughly coats the surface is sufficient and has no influence on the measurement result. Figure 3 shows a metal sample before and after coating with graphite.
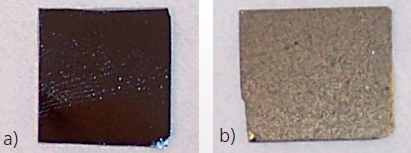
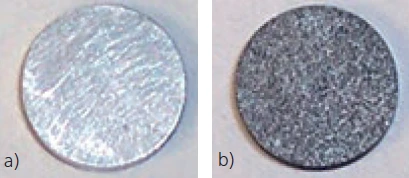
When sputtering gold onto very thin samples, only a thin gold layer of nm range thickness needs to be applied. The goal is to eliminate any light transmission through the sample. The adequacy of the gold coating in blocking light transmission can be checked with a strong light source. The sputtering process must be repeated until light is no longer transmitted by the sample. The gold-coated sample should then be dusted (not coated) with graphite such that the gold layer is still clearly visible. An example is presented in figure 4.
How Does the Coating Affect the Measurement Result?
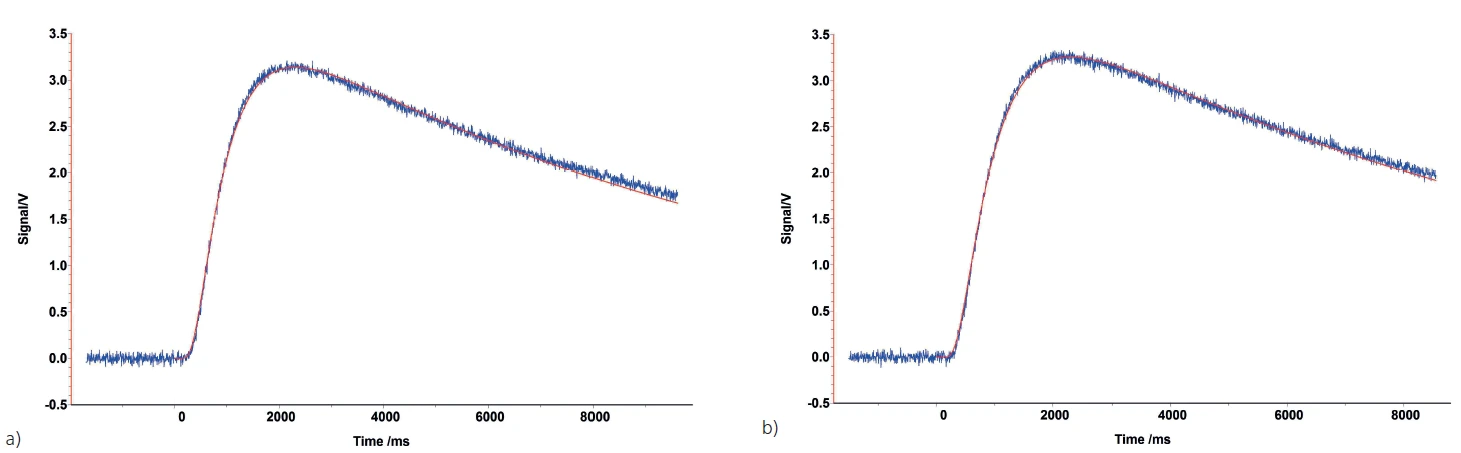
A correct applied coating has no influence on the measurement result. There are, however, a few exceptions where the coating should be applied with special care in order to avoid a negative impact on the measurement. For highly conducting materials, such as copper or aluminum, too thick of a graphite layer can shift the Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity of the samples to lower values since graphite is a poorer conductor. An example of this is shown in figure 5.
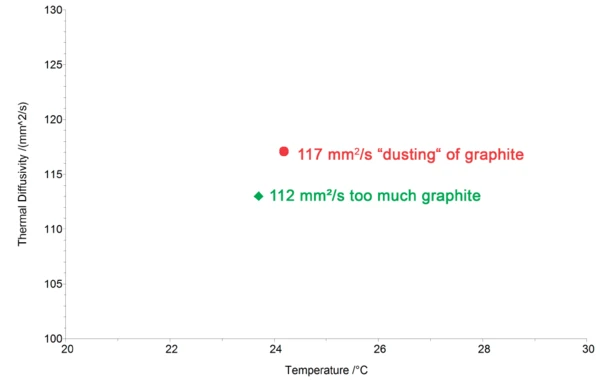
In this example, coating the copper sample with a graphite layer of normal thickness (approx. 5 μm) caused a 4% decrease in the Thermal ConductivityThermal conductivity (λ with the unit W/(m•K)) describes the transport of energy – in the form of heat – through a body of mass as the result of a temperature gradient (see fig. 1). According to the second law of thermodynamics, heat always flows in the direction of the lower temperature.thermal conductivity of the copper from its nominal value of 117 m²/s. When only a small “dusting” of graphite was applied (figure 6), the correct Thermal DiffusivityThermal diffusivity (a with the unit mm2/s) is a material-specific property for characterizing unsteady heat conduction. This value describes how quickly a material reacts to a change in temperature.thermal diffusivity value was obtained (red symbol in the graph).
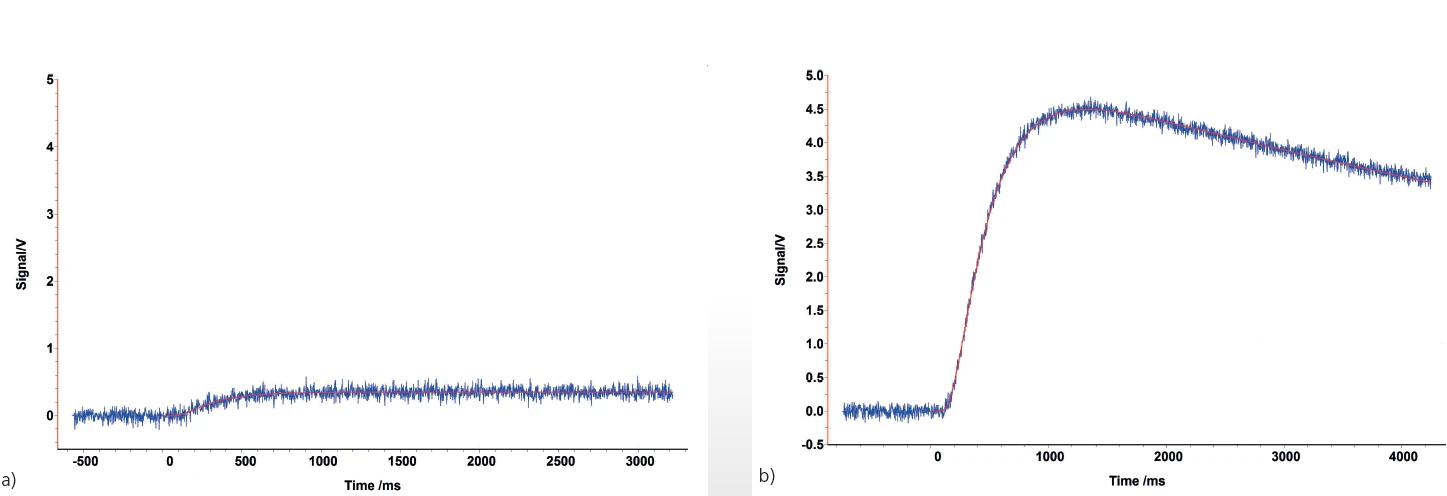
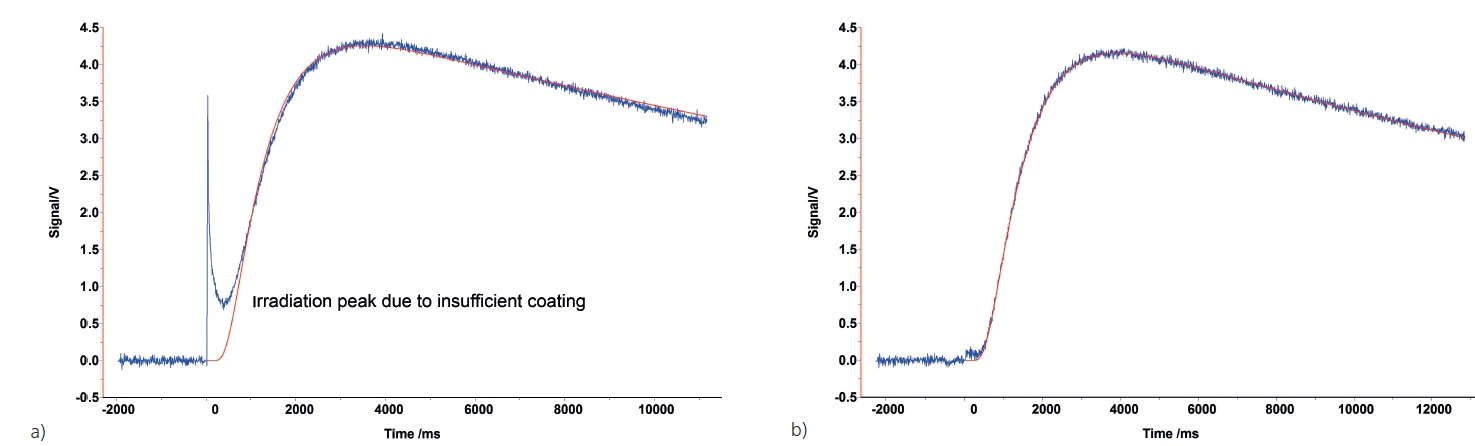
It is also possible to apply too little graphite. This can happen, for example, with some polymers. As shown at the beginning of the measurement in figure 7a), if the graphite coating is too thin, radiation from the flash lamp can penetrate to the detector. In this case, it is advisable to apply a coating that is thick enough to prevent this light penetration, as shown in figure 7b).
Conclusion
In general, all samples should be coated to some extent prior to an LFA measurement. Depending on the type and thickness of the material to be tested, e.g., gold and/or graphite, can serve as coating materials. A simple graphite layer is most often sufficient. The thickness of the graphite layer that should be used will depend on the thickness and conductivity of the sample and on whether or not a gold coating is employed.