Introduction
Amoxicillin (figure 1) is an antibiotic from the aminopenicillin group. It is used for the treatment of bacterial infections such as middle ear infections, pneumonia, and skin infections [2]. Here, it was measured by means of DSC and TG-FT-IR in order to obtain information about some of its thermal properties such as Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting point and degradation temperature along with degradation products.
Test Results
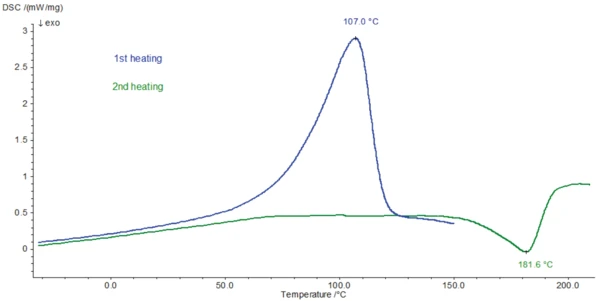
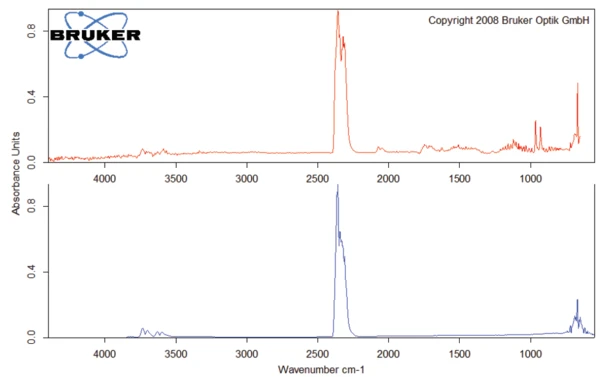
The DSC measurement was carried out on an amoxicillin trihydrate sample (1.622 mg) with the DSC 204 F1 Phoenix®. An aluminum crucible with a manually pierced lid (3 holes) was used. The sample was heated twice from -80°C at a heating rate of 10 K/min; the first time to 150°C, the second time to 210°C. Between the two heating runs, the sample was cooled at a controlled rate of 10 K/min. The DSC measurement of the heating runs is depicted in figure 2.
For the TGA measurement, a sample (4.79 mg) was prepared in an aluminum oxide crucible and heated to 700°C in a dynamic nitrogen atmosphere at 10 K/min. The TGA curve is depicted in figure 3.
The endothermal peak detected at 107°C (DSC curve, 1st heating, figure 2) is associated with a mass loss of 12.9%. This process − which can be related to the release of volatiles − is recorded at a higher temperature in the DSC measurement than the corresponding mass loss in the TGA curve. The temperature at which the evaporation occurs is dependent on the presence of a lid on the crucible and on the size of the holes in the crucible lid. Since no lid was used for the TGA measurement, volatilization occurs at a lower temperature than for the DSC measurement, where a pierced lid is employed.
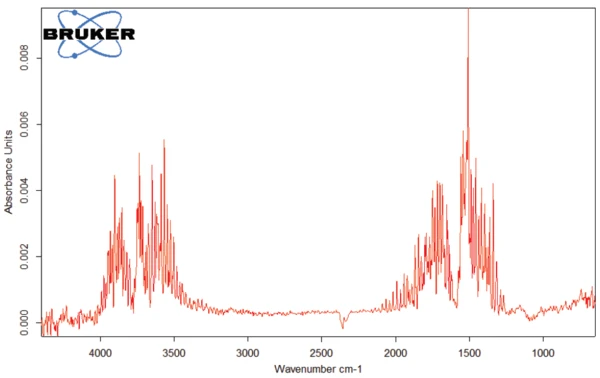
The FT-IR spectrum of the products released at 93°C (figure 4) is characteristic for water.
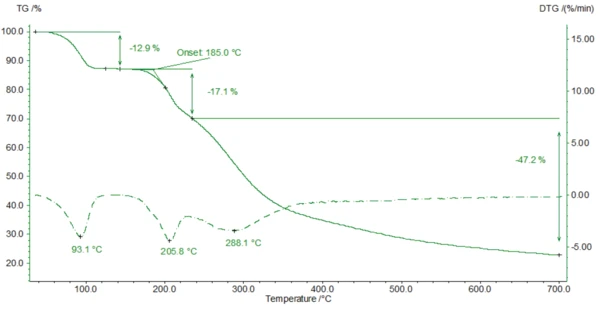
Amoxicillin has a molar mass of 365.4 g/mol [2]. This results in a molar mass of amoxicillin trihydrate of 419.4 g/mol. Consequently, the release of all of the water from amoxicillin trihydrate would theoretically yield a mass change of about 12.9%. Here (figure 3), the measurement results are in excellent accordance with the theoretical values.
A 2nd mass-loss step occurs at 185°C (onset value of the TGA curve). The corresponding FT-IR spectra reveal that degradation of amoxicillin begins with the release of carbon dioxide (figure 5) and ammonia (figure 6). It is associated with an ExotérmicoA sample transition or a reaction is exothermic if heat is generated.exothermal effect in the DSC curve. Degradation continues such that amoxicillin loses more than 77% of its initial mass during heating to 700°C.
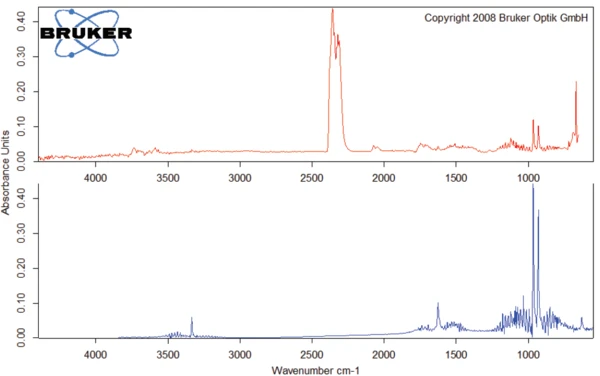
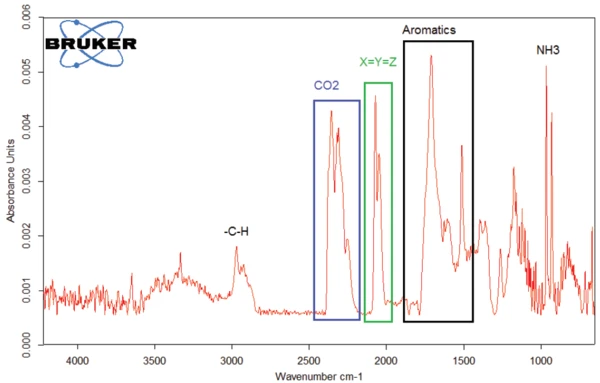
The FT-IR spectrum for the substances released at 294°C is given in figure 7. The characteristic bands for CO2 and NH3, which were already discussed, were found as well. However, there are additional absorbance bands visible: The bands between 3000 and 2800 cm-1 indicate the presence of –C-H bonds, whereas the bands found between 1500 and 1800 cm-1 can result from aromatics. The domain between 1900 and 2300 cm-1 is typical for triple bonds or double bonds X_Y_Z. [3, 4] .
Summary
The effects detected by means of DSC measurements during the thermal treatment of amoxicillin cannot solely be explained by interpretation of the DSC results. Only by additional measurement with the TGA-FT-IR method combination can be confirmed that the DSC effect at 107°C ist due to the evaporation of water and not to melting, causing an endothermal DSC peak, but no mass loss. The mass loss continuing after the evaporation of water is a result of the Reacción de DecomposiciónA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition. The Reacción de DecomposiciónA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition products, carbon dioxide and ammonia among others, can clearly be identified with the help of the FT-IR.