04.05.2020 by Claire Strasser
Thermal Stability of Drugs
The thermal stability of a substance is closely related to the shelf-life of pharmaceutical products. Here, the thermal stability of diclofenac sodium (anti-inflammatory) is determined by means of thermogravimetry.
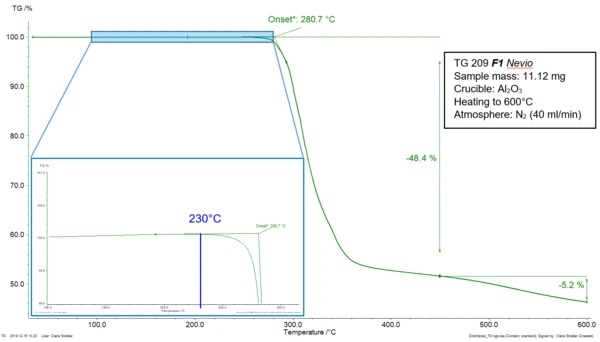
A material is thermally stable as long as no change occurs in its structure and properties when submitted to higher temperatures. Standard ASTM E2550-17 describes the thermal stability of a substance as “the temperature at which [it starts] to decompose or react” [1] during a thermogravimetric measurement. In this technique, the influence of the temperature on the behavior of a sample is determined. For that, “the mass of a substance is measured as a function of temperature or time while the substance is subjected to a controlled-temperature program in a specified atmosphere.” [2] Figure 1 depicts the TGA measurement of diclofenac sodium during heating at 10 K/min in a nitrogen atmosphere. The mass-loss step of the curve can be characterized by the extrapolated onset temperature at 281°C as described in ISO 11358-1 [3]. In ASTM E2550-17, the beginning of the step is determined by “the point on the TG curve where a deflection is first observed from the established baseline prior to the thermal event.” [1] In the given example, it is located at 230°C (blue indication in the zoomed area). The mass loss occurring at 281°C (extrapolated onset temperature, ISO 11358-1) and 230°C (onset temperature, ASTM E2550-17), is related to the thermal Réaction de DécompositionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition of the sample.
You will find more information about thermal stability measurements here.