Pharma
Potassium Clavulanate - Thermal stability
The potassium salt of clavulanic acid is an active pharmaceutical ingredient usually administered in combination with amoxicillin, an antibiotic substance. It is less hygroscopic than clavulanic acid, but still absorbs some water.
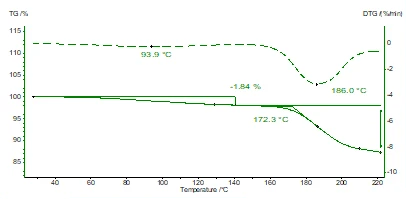
Although the substance fresh from the bottle was designated as dry, there is a small mass loss (identified as water in the corresponding FT-IR spectra, not shown here) of approx. 1.8% which starts to evaporate already around 60°C. Degradation of the potassium clavulanate itself starts at about 150/160°C.
Thermal stability experiments are usually carried out under inert conditions. According to ASTM E2550, a material is stable up to the temperature at which it starts to decompose or react.
The conditions for the TGA measurements were: sample weight: 5.34 mg, heating rate: 10 K/min, nitrogen atmosphere, Al crucibles (hermetically sealed and pierced shortly before inserting inside of the instrument).