Glossary
Vaporization
The Vaporization of an element or compound is a Phase TransitionsThe term phase transition (or phase change) is most commonly used to describe transitions between the solid, liquid and gaseous states.phase transition from the liquid phase to vapor. There exists two types of vaporization: evaporation and boiling.
Evaporation is a surface phenomenon and only occurs on the phase boundary between the liquid and the gaseous phase. The surface atoms or molecules gains energy from surroundings and overcome the attractions of other molecules and get vaporized. Evaporation only occurs when the partial pressure of the vapor of a substance is less than the equilibrium vapor pressure.
Boiling describes the bulk phenomenon of the rapid vaporization of a liquid at its boiling point. The entire bulk of liquid and all the molecules including the interior and the surface gain energy to change to vapor state. Boiling occurs when the equilibrium vapor pressure of the substance is greater than or equal to the environmental pressure. The temperature at which boiling occurs is the boiling temperature, or boiling point. The boiling point varies with the pressure of the environment.
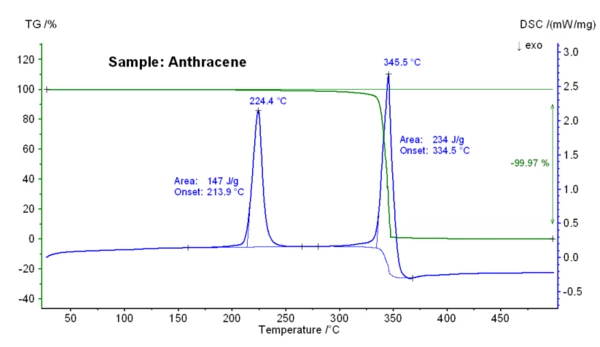
This figure below depicts the temperature-dependent mass changes and the DSC signal of an anthracene sample. At an extrapolated onset temperature of 213.9°C, an EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic DSC effect with an enthalpy of 147 J/g was detected; this indicates the melting of anthracene. A mass-loss step of 100% occurred between 250°C and 375°C which is the result of the vaporization of the sample. Initially, only a small mass loss is shown by the evaporation of the sample. However, upon reaching the boiling point temperature (onset 334 ° C) the rapid and complete evaporation occurs by boiling anthracene sample.
This figure depicts the temperature-dependent mass changes and the DSC signal of an anthracene sample. At an extrapolated onset temperature of 213.9°C, an EndothermicA sample transition or a reaction is endothermic if heat is needed for the conversion.endothermic DSC effect with an enthalpy of 147 J/g was detected; this indicates the melting of anthracene. A mass-loss step of 100% occurred between 250°C and 375°C which is the result of the vaporization of the sample. Initially, only a small mass loss is shown by the evaporation of the sample. However, upon reaching the boiling point temperature (onset 334 ° C) the rapid and complete evaporation occurs by boiling anthracene sample.