Introduction
Bentonite is a clay consisting mainly of montmorrillonite and stands out due to its absorbent capabilities. Some of the main uses are as a binder, purifier, absorbent and as a groundwater barrier.

Measurement Conditions and Results
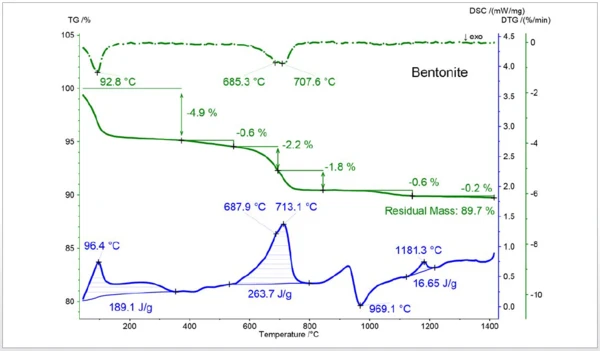
This plot exhibits the TGA (green), DTGA (green dotted) and DSC (blue) curves of a 19.7 mg bentonite sample. The 1st mass-loss step (DSC peak temperature 96°C) is due to the release of water followed by a small mass-loss step of 0.6%. This is most likely due to the release of SO2 indicating a pyrite contamination. Above 600°C, water is released from the bentonite structure (DTGA peaks at 685°C and 708°C). The exothermic DSC peak at 969°C represents the phase transition of this clay mineral. The endothermic DSC peak at 1181°C is most likely due to partial melting or a further SO2 release.
Conclusion
Complex thermal behavior can be investigated with the STA 449 F5 Jupiter®. In case of inhomogeneous mixtures, crucibles with high volumes are available which allow for an increased sample mass of several grams.