Introduction
Parts made of polymer materials are widely used in all areas where weight reduction and cost-effective production play a decisive role. Although injection molding parts made of thermoplastic materials have been used in the automotive industry for decades, the demand for lightweight solutions for modern automobiles continues to increase. Especially for the development of electric vehicles and for lowering CO2 emissions, more and more automotive components made of light materials are being used.
The increased use of plastics necessitates a means of ensuring consistent quality and stability of the parts. Here, material analysis plays a major role. The mechanical properties of parts are significantly influenced by many process steps. For example, simply painting a plastic can change its physical properties to the extent that, in the Worst-Case ScenarioRelated to a chemical reactor, a worst-case scenario is the situation where temperature and/or pressure production caused by the reaction runs out of control.worst case scenario, it will fail when exposed to a reasonable load. Therefore, it is important to guarantee the constant quality of materials throughout the manufacturing process, from beginning to end. Thermal analysis methods, such as differential scanning calorimetry (DSC) are ideal tools for issues such as these. In the case considered herein, a housing component made of glass fiber-reinforced Polyamide 6 showed embrittlement at the clip hook during connection with the clip joints. During installation of the part, the clip broke. For such failures, it is crucial to examine all potential influencing factors throughout the manufacturing chain.
Test Results
DSC analysis of the damaged part and an iO control part quickly identified the cause of the failure. The DSC curves are presented in figure 1. For analysis of the material composition, the 2nd heating curves are always evaluated since any effects of the thermal history are no longer present. Along with the Glass Transition TemperatureThe glass transition is one of the most important properties of amorphous and semi-crystalline materials, e.g., inorganic glasses, amorphous metals, polymers, pharmaceuticals and food ingredients, etc., and describes the temperature region where the mechanical properties of the materials change from hard and brittle to more soft, deformable or rubbery.glass transition of the sample at 50.9°C, the control part (green curve) showed a melting endotherm at 221°C with a melting enthalpy of 53.7 J/g (typical for pure PA 6). The niO part (damaged), however, showed measurably different behavior, with a peak temperature at 215°C and an enthalpy of 45.2 J/g.
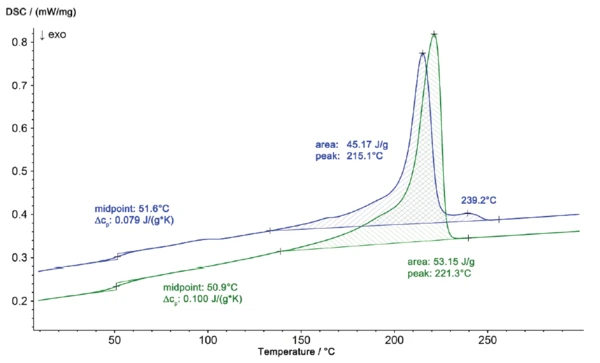
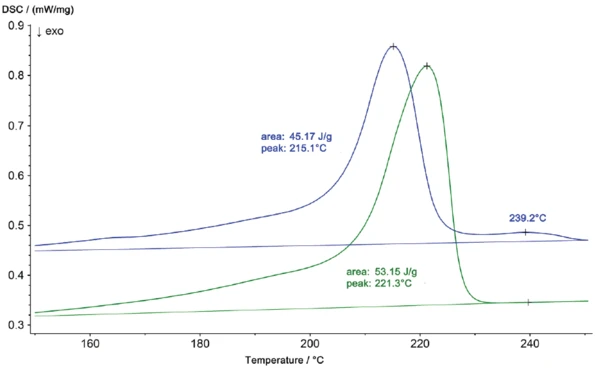
The melting profile of the niO part, shown on an enlarged scaling in figure 2, also shows a second peak at 239°C. The results of the DSC measurements reveal that the material of the damaged part is no longer pure Polyamide 6 but, rather, a mixture of Polyamide 6 and Polyamide 66. These two components can form a eutectic, which accounts for the shift in the Melting Temperatures and EnthalpiesThe enthalpy of fusion of a substance, also known as latent heat, is a measure of the energy input, typically heat, which is necessary to convert a substance from solid to liquid state. The melting point of a substance is the temperature at which it changes state from solid (crystalline) to liquid (isotropic melt).melting temperature from 221°C (pure PA 6) to 215°C (PA 6 + PA 66). The differences between the two samples can also be seen from their different CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization profiles during cooling (figure 3.)
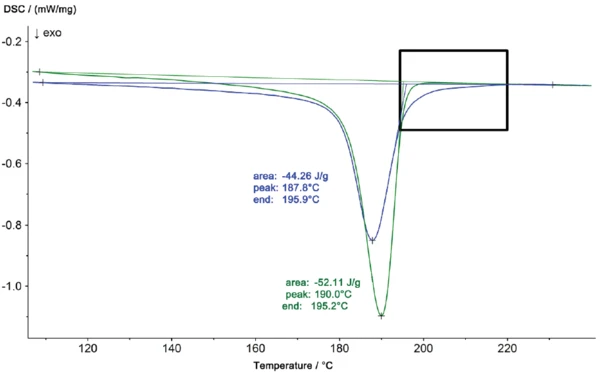
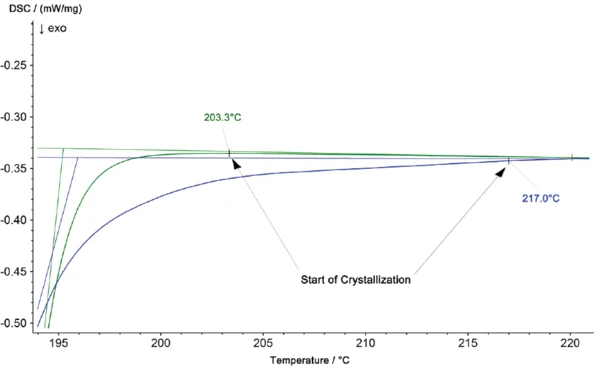
In the DSC analysis, CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization is observed as an ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic effect. The enlarged scaling in figure 4 further shows a higher onset temperature for CrystallizationCrystallization is the physical process of hardening during the formation and growth of crystals. During this process, heat of crystallization is released.crystallization of the material from the iO part at 217°C, compared to 203°C for the pure PA6 sample. The area of the peak is also smaller for the iO part.
Conclusion
This example clearly demonstrates that the material composition has a measurable influence on the properties of a finished part and that failures can be avoided by using thermal analysis to monitor the quality of the raw material. Quality control can be accomplished with relatively little effort using thermal analysis by means of DSC.