
Hydrogen Peroxide
Pure hydrogen peroxide (H2O2) is a pale blue liquid, mixable in any ratio with water. Low-percentage aqueous solutions are widely used as bleaching agents due to their strong oxidizing properties. Besides for the bleaching of wood, paper or hair, hydrogen peroxide solutions are also used as oxidizing agents or in medical application as disinfectants. The tendency of hydrogen peroxide to decompose into water and oxygen (equation 1 below) is the reason for its application as a liquid propellant in rocket engines.
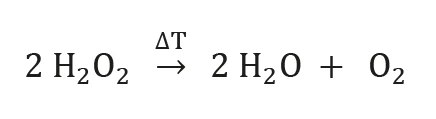
The Multiple Module Calorimeter (MMC) Compared to Differential Scanning Calorimetry (DSC)
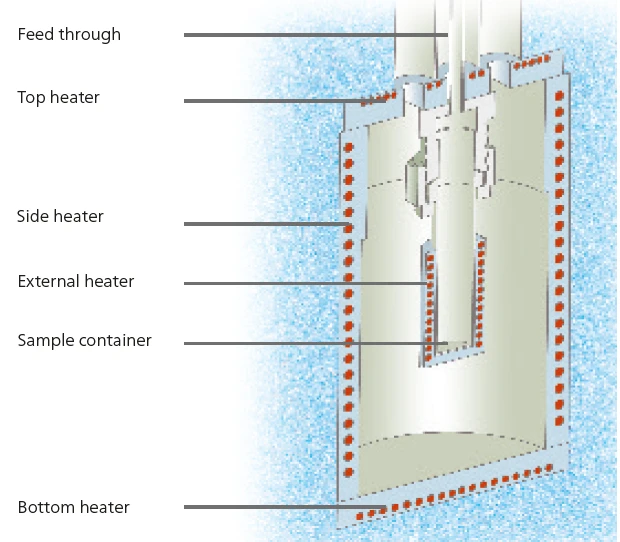
The NETZSCH Multiple Module Calorimeter Multiple Module Calorimeter (MMC)A multiple mode calorimeter device consisting of a base unit and exchangeable modules. One module is prepared for accelerating rate calorimetry (ARC), the ARC-Module. A second one is used for scanning tests (Scanning Module) and a third one is related to battery testing for coin cells (Coin Cell Module).MMC 274 Nexus® (figure 1) offers three different measurement modules [1]. The Accelerating Rate Calorimetry (ARC)The method describing isothermal and adiabatic test procedures used to detect thermally exothermic decomposition reactions.ARC® Module can be used for thermal hazard studies; the Coin-Cell Module is specialized for the investigation of batteries; and the Scanning ModuleA calorimeter module being part of the Multipe Module Calorimeter (MMC) allowing for scanning test of a sample. This procedure can serve as a screening test in order to detect a thermal hazard potential within a reasonably short measurement time.Scanning Module can be used to evaluate caloric data from a single heating run. In contrast to the widely used and well-known technique of differential scanning calorimetry (DSC), the Scanning ModuleA calorimeter module being part of the Multipe Module Calorimeter (MMC) allowing for scanning test of a sample. This procedure can serve as a screening test in order to detect a thermal hazard potential within a reasonably short measurement time.Scanning Module of the Multiple Module Calorimeter (MMC)A multiple mode calorimeter device consisting of a base unit and exchangeable modules. One module is prepared for accelerating rate calorimetry (ARC), the ARC-Module. A second one is used for scanning tests (Scanning Module) and a third one is related to battery testing for coin cells (Coin Cell Module).MMC can handle samples up to a volume of 2 ml. For heating the samples, there are two options available: either a constant heating rate or a constant level of power. By using information about both the power supplied to the sample and the heating rate, a heat-flow signal can be calculated. Using metals such as indium, tin and bismuth, both the temperature and the sensitivity of the instrument can be determined. At 1000 to 9000 mg (sample volume about 1 ml), typical sample masses are considerably higher for the Multiple Module Calorimeter (MMC)A multiple mode calorimeter device consisting of a base unit and exchangeable modules. One module is prepared for accelerating rate calorimetry (ARC), the ARC-Module. A second one is used for scanning tests (Scanning Module) and a third one is related to battery testing for coin cells (Coin Cell Module).MMC than sample masses used for DSC, which are typically between 5 and 10 mg. Even so, the evaluated uncertainty for the Scanning ModuleA calorimeter module being part of the Multipe Module Calorimeter (MMC) allowing for scanning test of a sample. This procedure can serve as a screening test in order to detect a thermal hazard potential within a reasonably short measurement time.Scanning Module of the Multiple Module Calorimeter (MMC)A multiple mode calorimeter device consisting of a base unit and exchangeable modules. One module is prepared for accelerating rate calorimetry (ARC), the ARC-Module. A second one is used for scanning tests (Scanning Module) and a third one is related to battery testing for coin cells (Coin Cell Module).MMC is about 1% for temperature determinations and less than 5% for enthalpy determinations.
Scanning Module and ARC® Module
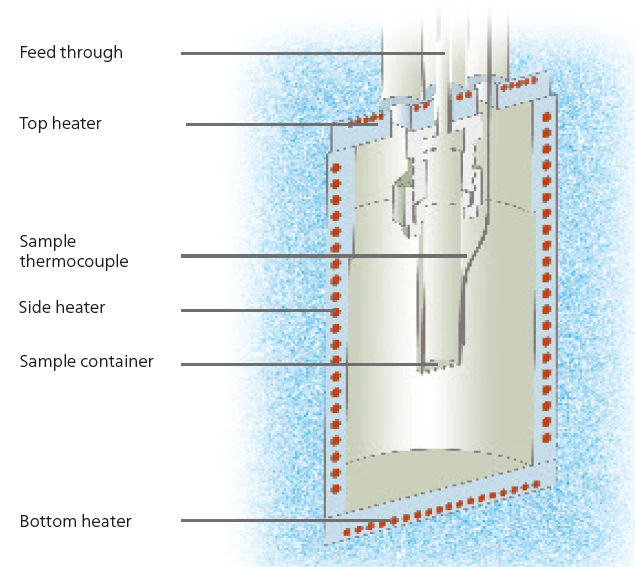
This work studies the thermal Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition behavior of aqueous hydrogen peroxide solutions of varying concentrations. Two MMC modules are employed for these studies: the Scanning ModuleA calorimeter module being part of the Multipe Module Calorimeter (MMC) allowing for scanning test of a sample. This procedure can serve as a screening test in order to detect a thermal hazard potential within a reasonably short measurement time.Scanning Module (see figure 2) for screening of the samples and the Accelerating Rate Calorimetry (ARC)The method describing isothermal and adiabatic test procedures used to detect thermally exothermic decomposition reactions.ARC® Module (see figure 3) for Heat-Wait-Search (HWS)Heat-Wait-Search is a measurement mode used in calorimeter devices according to accelerating rate calorimetry (ARC).heat-wait-search (Heat-Wait-Search (HWS)Heat-Wait-Search is a measurement mode used in calorimeter devices according to accelerating rate calorimetry (ARC).HWS) studies. Via an external heater which directly surrounds the sample vessel (figure 4), the Scanning ModuleA calorimeter module being part of the Multipe Module Calorimeter (MMC) allowing for scanning test of a sample. This procedure can serve as a screening test in order to detect a thermal hazard potential within a reasonably short measurement time.Scanning Module can provide the sample with a constant level of power.

Measurement Conditions
Hydrogen peroxide (Sigma Aldrich) was received as an aqueous solution (35%) and stored at ambient temperature. The hydrogen peroxide solution was used as received and was diluted with purified water in order to observe several lower concentrations. The composition of the diluted samples is summarized in table 1 and table 2. The measurement conditions for both the Scanning and Accelerating Rate Calorimetry (ARC)The method describing isothermal and adiabatic test procedures used to detect thermally exothermic decomposition reactions.ARC® Modules are compared in table 3.
Tab 1: Sample compositions for screening (Scanning Module)
| Sample Number | Sample Concentration/% | H2O2/g | H2O/g | Total/g |
|---|---|---|---|---|
| 1 | 35 | 1.03106 | 0.0 | 1.03106 |
| 2 | 26 | 0.75757 | 0.25623 | 1.0138 |
| 3 | 17 | 0.5148 | 0.52494 | 1.03974 |
| 4 | 8.6 | 0.25169 | 0.7741 | 1.02579 |
| 5 | 4.3 | 0.12376 | 0.88605 | 1.00981 |
| 6 | 2.6 | 0.07316 | 0.92551 | 0.99867 |
| 7 | 1.1 | 0.03099 | 0.96707 | 0.99806 |
| 8 | 0.4 | 0.01215 | 1.00176 | 1.01391 |
Tab 2: Sample compositions for AdiabaticAdiabatic describes a system or measurement mode without any heat exchange with the surroundings. This mode can be realized using a calorimeter device according to the method of accelerating rate calorimetry (ARC). The main purpose of such a device is to study scenarios and thermal runaway reactions. A short description of the adiabatic mode is “no heat in – no heat out”.adiabatic testing (Accelerating Rate Calorimetry (ARC)The method describing isothermal and adiabatic test procedures used to detect thermally exothermic decomposition reactions.ARC® Module)
| Sample Number | Sample Concentration/% | H2O2/g | H2O/g | Total/g |
|---|---|---|---|---|
| 9 | 35 | 1.02157 | 0.0 | 1.02157 |
| 10 | 17 | 0.74935 | 0.52494 | 1.00359 |
| 11 | 8.6 | 0.51466 | 0.50962 | 1.02428 |
| 12 | 4.3 | 0.25036 | 0.77525 | 1.02561 |
| 13 | 2.6 | 0.14776 | 0.877248 | 1.02034 |
Tab 3: Measurement conditions
MMC 274 Nexus® | ||
|---|---|---|
| MMC Module | Scanning | ARC® |
| Vessel material | Stainless steel | Stainless steel |
| Vessel type | Closed | Closed |
| Vessel mass | 7.0 to 7.25 g | 7.0 to 7.25 g |
| Heating | Constant power (250 mW) | |
| Atmosphere | Air | Air |
| Purges gas rate | Static | Static |
| Temperature range | RT ... 250°C | RT ... 250°C |
| Sample mass | 998.67 to 1039.74 mg | 1003.6 to 1025.6 mg |
Results and Discussion
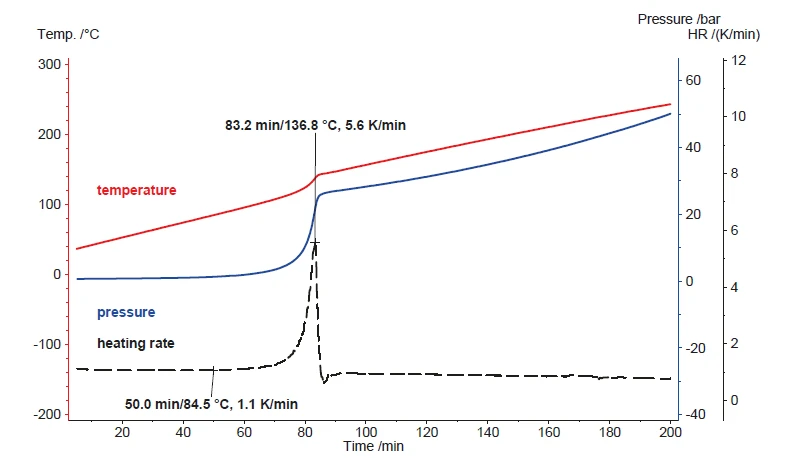
Depending on the change in the samples’ heat capacity, the constant power input usually results in an almost constant heating rate at the sample. Figure 5 shows the outcome of heating hydrogen peroxide (35%) using the Scanning Module at a constant power input of 250 mW. The resulting heating rate is about 1 K/min for the first 60 minutes. After one hour, the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition reaction starts and produces additional heat. During the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition reaction, the heating rate rises to a maximum of 5.6 K/min and the detected pressure rises as well. According to equation 1, the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition reaction generates oxygen. Besides the evaporation of water, this gas formation is the major reason for the pressure increase during heating.
Comparison of the Behavior of H2O2, H2O and Empty Vessel
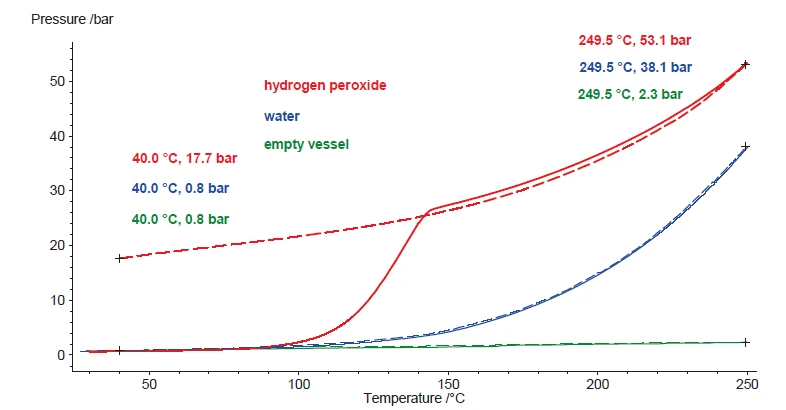
The results in figure 5 present exclusively the sample heating. Since the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition reaction of hydrogen peroxide is not reversible, the oxygen generated is not taken up again in order to form the initial hydrogen peroxide during cooling. Instead, the formed products of water and oxygen cool to ambient temperature as a liquid and a gas, respectively. The pressure signal indicates 17.7 bar at 40°C, which reflects the amount of oxygen being formed during Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition (figure 6). Taking the same amount of water instead, the pressure also increases during heating, but since water remains chemically unchanged, all water vapor precipitates again during cooling. That’s why the dashed blue line, indicating the pressure signal for water during cooling, shows values almost identical to the heating (solid lines). Just for comparison, the green lines show the course of the pressure signal during heating and cooling for an empty vessel.
H2O2 with Various Concentrations
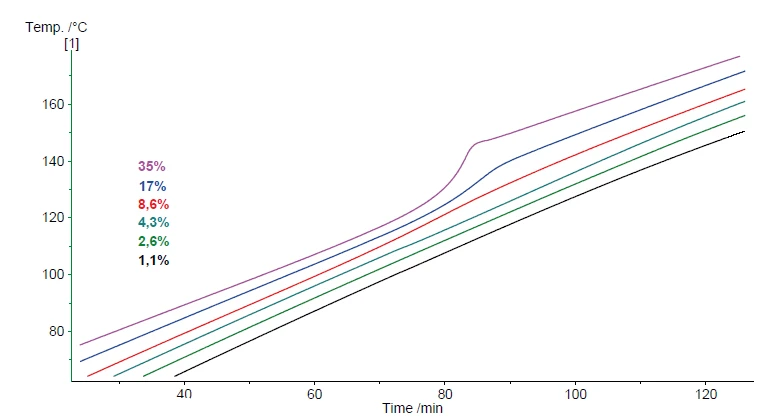
Especially when comparing with water, it can be seen that evaporation – which occurs to some extent even inside a closed vessel system – is always reversible. This is confirmed by the pressure signal at 40°C after the cool-down. On the other hand, the Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition reaction of hydrogen peroxide produces a specific amount of gas. Therefore, the pressure signal is expected to be proportional to the absolute amount of hydrogen peroxide inside the solution. When repeating these tests with samples of various hydrogen peroxide concentrations, the pressure build-up during the test should be proportional to the hydrogen peroxide concentration. Figure 7 compares the heating results for samples 1 to 6. The associated hydrogen peroxide concentrations are summarized in table 1.
Correlation Between H2O2 Concentration and Pressure
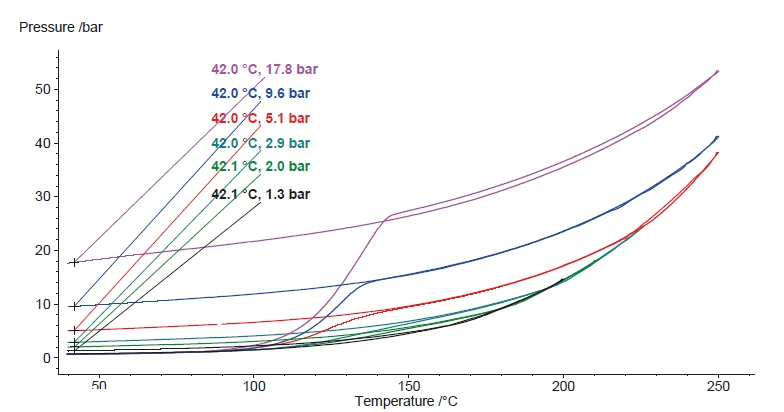
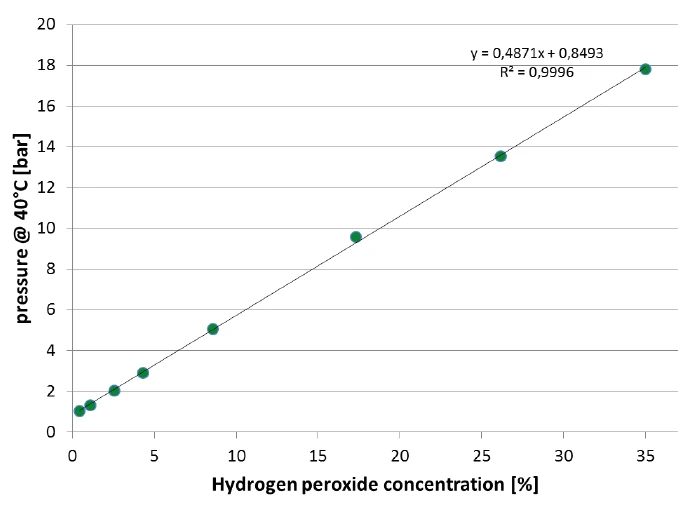
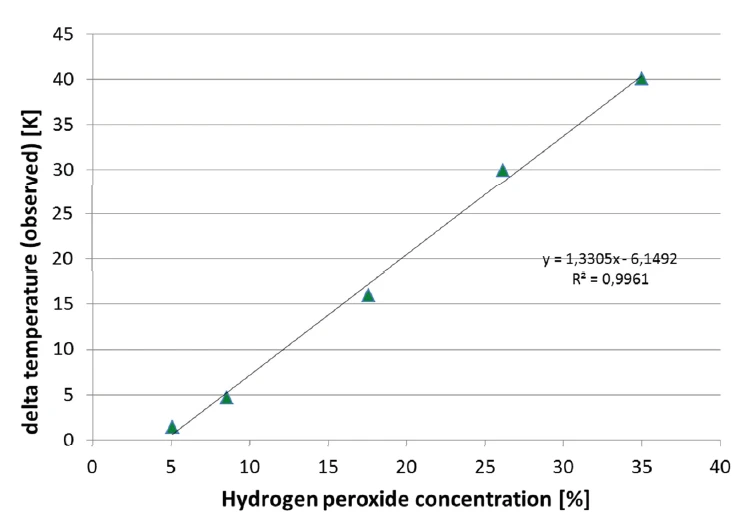
The Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition reaction of hydrogen peroxide is indicated by the increase in heating rate measured at the sample as well as by the build-up of pressure. In figure 8, the signal of the remaining pressure after the reaction and after the cool-down to 42°C is evaluated. There is an almost perfectly linear correlation of the pressure with the hydrogen peroxide concentration of the sample. This correlation is depicted in figure 9.
Various concentrations of H2O2 investigated with theARC® Module
Various concentrations of H2O2 investigated with the ARC® ModuleA similar series of aqueous hydrogen peroxide concentrations was also investigated using the MMC’s ARC® Module (figure 3). The associated hydrogen peroxide concentrations are summarized in table 2. The ARC® Module can be employed to specifically determine the temperature of the beginning of Decomposition reactionA decomposition reaction is a thermally induced reaction of a chemical compound forming solid and/or gaseous products. decomposition by means of a socalled Heat-Wait-Search (HWS)Heat-Wait-Search is a measurement mode used in calorimeter devices according to accelerating rate calorimetry (ARC).heat-wait-search (Heat-Wait-Search (HWS)Heat-Wait-Search is a measurement mode used in calorimeter devices according to accelerating rate calorimetry (ARC).HWS) program. With the help of the sequence of heating, equilibration and detection, the Self-heating rateA special kind of calorimeter device is used in order to detect the self-heating rate of a substance. The related method is called accelerating rate calorimetry (ARC). self-heating rate of the sample is determined under quasi-IsothermalTests at controlled and constant temperature are called isothermal.isothermal conditions and then, the sample is investigated in AdiabaticAdiabatic describes a system or measurement mode without any heat exchange with the surroundings. This mode can be realized using a calorimeter device according to the method of accelerating rate calorimetry (ARC). The main purpose of such a device is to study scenarios and thermal runaway reactions. A short description of the adiabatic mode is “no heat in – no heat out”.adiabatic mode [1, 2].
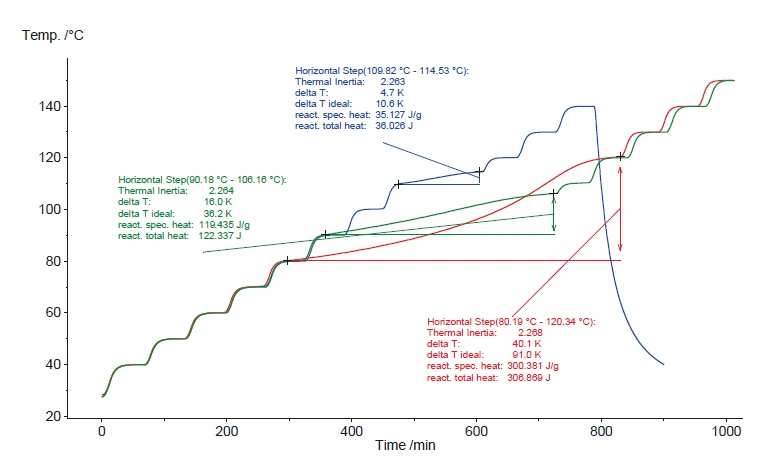
The results for hydrogen peroxide concentrations of 35%, 17% and 8.6% are presented in figure 10. As expected, the results confirm a smaller temperature increase (ΔTobs) under AdiabaticAdiabatic describes a system or measurement mode without any heat exchange with the surroundings. This mode can be realized using a calorimeter device according to the method of accelerating rate calorimetry (ARC). The main purpose of such a device is to study scenarios and thermal runaway reactions. A short description of the adiabatic mode is “no heat in – no heat out”.adiabatic conditions for lower hydrogen peroxide concentrations. The temperature at which the decomposition reaction is detected (onset) increases for lower concentrations due to the lower release of energy (90°C and 110°C). The maximum selfheating rate for hydrogen peroxide concentrations lower than 5% is less than 0.02 K/min. That’s why no ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic events are detected in such a case. The temperature increase steps (ΔTobs) detected during the AdiabaticAdiabatic describes a system or measurement mode without any heat exchange with the surroundings. This mode can be realized using a calorimeter device according to the method of accelerating rate calorimetry (ARC). The main purpose of such a device is to study scenarios and thermal runaway reactions. A short description of the adiabatic mode is “no heat in – no heat out”.adiabatic segments of several Heat-Wait-Search (HWS)Heat-Wait-Search is a measurement mode used in calorimeter devices according to accelerating rate calorimetry (ARC).HWS tests are shown in figure 11.
Conclusion
These results nicely demonstrate the screening capability of the MMC Scanning Module. In the case of strongly ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic reactions, the Self-heating rateA special kind of calorimeter device is used in order to detect the self-heating rate of a substance. The related method is called accelerating rate calorimetry (ARC). self-heating rate will increase significantly – to above the level of roughly 1 K/min – as a result of the constant power input. Thus, when an unknown sample exhibits an ExothermicA sample transition or a reaction is exothermic if heat is generated.exothermic decomposition reaction, this can be recognized within several hours. As soon as hazardous potential is recognized, an AdiabaticAdiabatic describes a system or measurement mode without any heat exchange with the surroundings. This mode can be realized using a calorimeter device according to the method of accelerating rate calorimetry (ARC). The main purpose of such a device is to study scenarios and thermal runaway reactions. A short description of the adiabatic mode is “no heat in – no heat out”.adiabatic test is recommended using an MMC ARC® Module [1]. Such an HWS test can easily take a full day but on the other hand, is much more pertinent to thermal equilibrium than a scanning test [2].
Additionally, the results presented above nicely demonstrate the usefulness of the pressure signal. The constant power input of 250 mW enables a heating rate of approximately 1 K/min for an aqueous sample of 1 g. Samples having a concentration of hydrogen peroxide lower than 5% do not exceed this heating rate via the energy released during the decomposition reaction. This means that, through the Self-heating rateA special kind of calorimeter device is used in order to detect the self-heating rate of a substance. The related method is called accelerating rate calorimetry (ARC). self-heating rate of the sample, the decomposition reaction for low concentrations is masked by the power input. The pressure signal, in contrast, is unaffected by the power input. Therefore, it can be taken as a significant indicator as to whether or not a decomposition reaction has occurred, especially in the case of lower concentrations.