Introduction
Oxalates are the salts of the oxalic acid C2H2O4 (COOH)2 (ethanedicarboxylic acid). The calcium salt of oxalic acid, calcium oxalate, crystallizes in the anhydrous form and as a solvate with one molecule of water per formula, as calcium oxalate monohydrate CaC2O4*H2O.
Occurrence and Application
Although calcium oxalate monohydrate is the salt of an organic aicd, it can be found in nature as a primary mineral. Figure 1 shows a Whewellite crystal from the Schlema locality in Germany‘s Erzgebirge Mountain Range. In addition to Whewellite, weddellite is also known as a second mineral species [1].

Calcium oxalate is also the main component of kidney stones.
In thermal analysis, calcium oxalate monohydrate is used to check the functionality of thermobalances. This substance has good storage stability; it is not subject to change over time, nor does it have any tendency to adsorb humidity from the laboratory atmosphere. These features make it an ideal referene substance for use in checking the temperature-base functionality of a thermobalance.
Measurement Conditions
- Instrument
- TG 209 F1 Libra®
- Sample
- CaC2O4*H2O
- Sample weights
- 8.43 mg (black curve in figure 2) and
- 8.67 mg (red curve in figure 2)
- Crucible
- Al2O3
- Atmosphere
- Nitrogen
- Gas flow rate
- 40 ml/min
- Heating rate
- 10 K/min (black curve in figure 2) and
- 200 K/min (red curve in figure 2)
Thermogravimetry
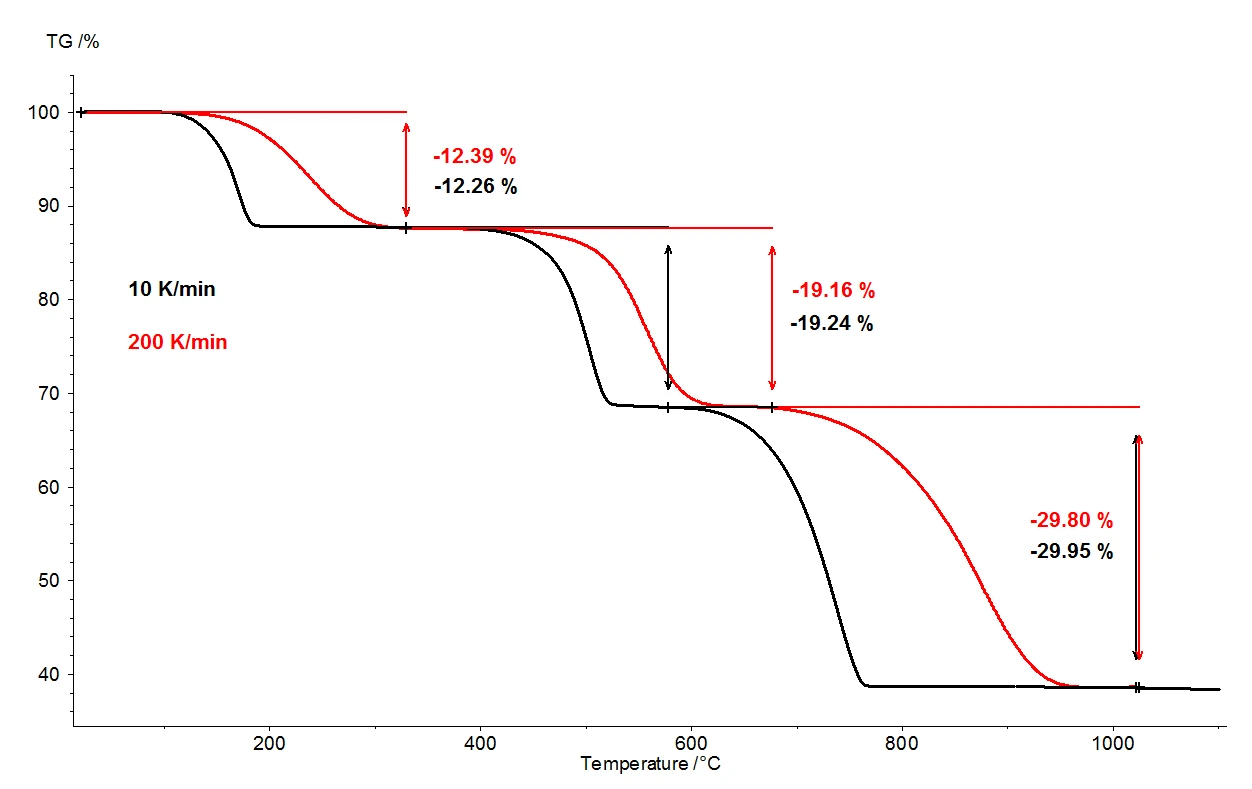
When calcium oxalate monohydrate is heated to 1100°C, three clearly separated mass-loss steps can be detected by means of the thermobalance. Figure 2 shows a comparison of the thermogravimetric results of two measurements on calcium oxalate monohydrate samples. The relative mass changes of the samples are recorded over the temperature. Presented in figure 3 is the analogous comparison of the two measurements, as a function of temperature, for the first derivative of thermogravimetric results (DTG).
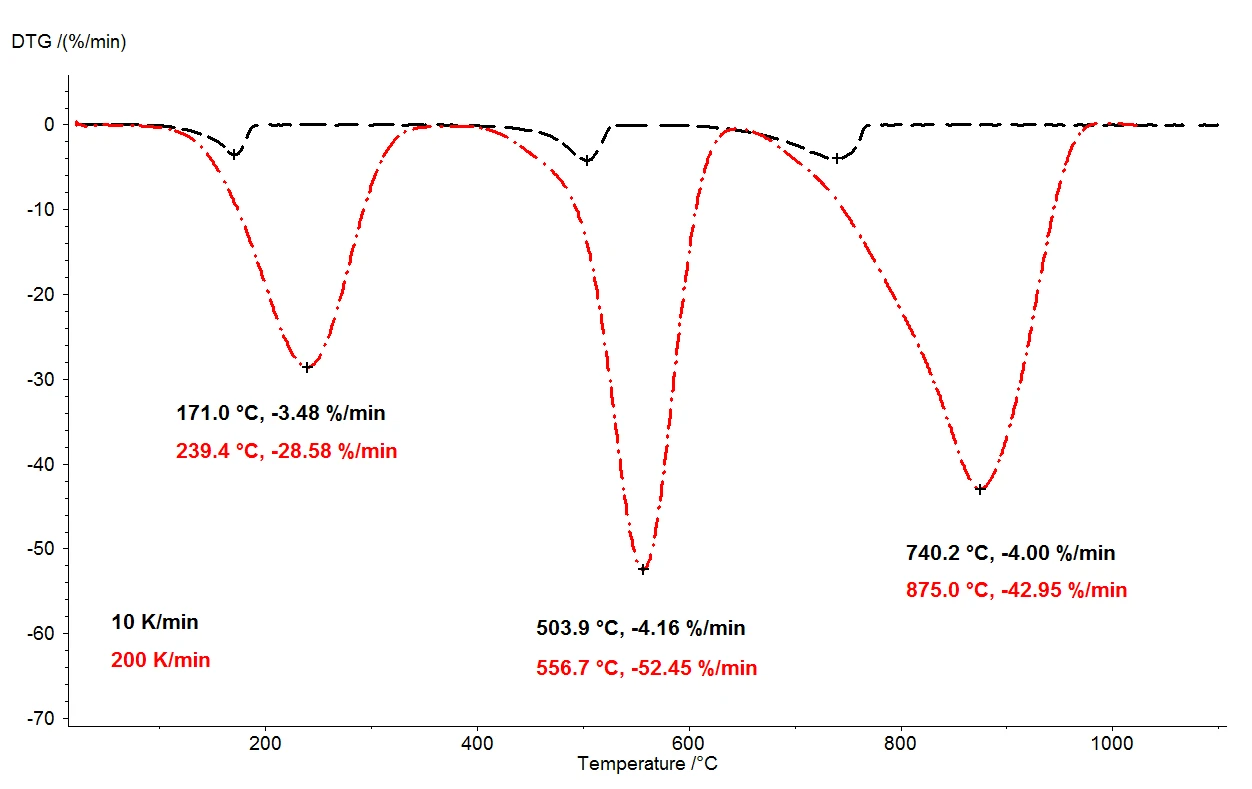
Under otherwise identical conditions, two different heating rates were selected: 10 K/min (black curve) and 200 K/ min (red curve). With the increased heating rate, the temperatures of the mass-loss steps are shifted to higher values and the release rates – the speed of the gas release – are increased approximately tenfold (DTG minima, figure 3). The shift in temperature that takes place upon variation of the heating rate is a well understood occurrence that can be applied toward further evaluation of the kinetic data [2]. Besides the temperature shift, it is also important to note that quantification of the mass-loss steps is independent of the heating rate. The heating rate of 200 K/min thus provides all the same information regarding the thermal degradation of calcium oxalate monohydrate as the more usual heating rate of 10 K/min; no information is lost by accelerating the heating rate. While yielding the same information content, however, the faster heating rate results in tremendous time savings: a measurement at 10 K/min takes almost two hours to cover the temperature range from room temperature to 1100°C, but the same measurement at 200 K/min is completed already in five minutes.
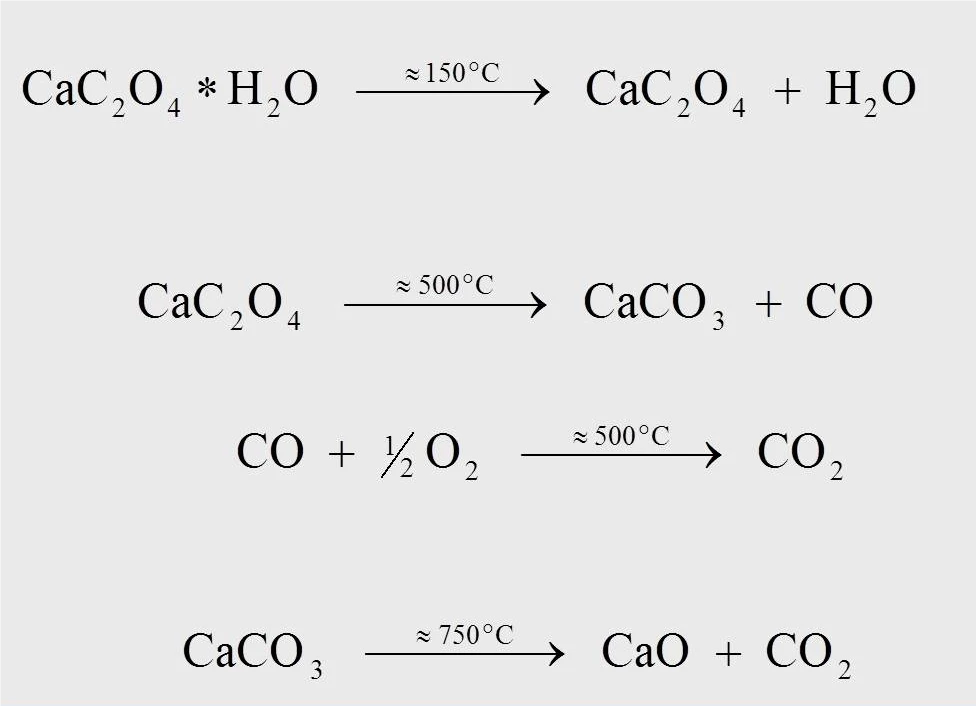
The reaction equations for the thermal degradation reaction of calcium oxalate monohydrate are shown in figure 4. At approximately 170°C, for the measurement at 10 K/min, anhydrous calcium oxalate is formed when water separates from the monohydrate (1). At approximately 500°C, calcium oxalate is transformed into calcium carbonate (CaCO3), and carbon monoxide (CO) splits off (2). The subsequent reaction, where the released carbon monoxide is oxidized to carbon dioxide (CO2) (3), can only occur in an oxygencontaining purge gas flow (e.g., synthetic air or oxygen). At a temperature of 750°C, calcium carbonate decomposes into calcium oxide with the release of CO2 (4).